Novel Chitosan-Coated Liposomes Coloaded with Exemestane and Genistein for an Effective Breast Cancer Therapy
- PMID: 38434864
- PMCID: PMC10905587
- DOI: 10.1021/acsomega.3c09948
Novel Chitosan-Coated Liposomes Coloaded with Exemestane and Genistein for an Effective Breast Cancer Therapy
Abstract
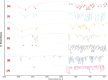
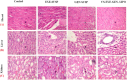
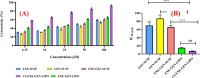
For achieving high effectiveness in the management of breast cancer, coadministration of drugs has attracted a lot of interest as a mode of therapy when compared to a single chemotherapeutic agent that often results in reduced therapeutic end results. Owing to their proven effectiveness, good patient compliance, and lower costs, oral anticancer drugs have received much attention. In the present work, we formulated the chitosan-coated nanoliposomes loaded with two lipophilic agents, namely, exemestane (EXE) and genistein (GEN). The formulation was prepared using the ethanol injection method, which is considered a simple method for getting the nanoliposomes. The formulation was optimized using Box-Behnken design (BBD) and was extensively characterized for particle size, ζ-potential, Fourier transform infrared (FTIR), differential scanning calorimetry (DSC), and X-ray diffraction (XRD) analysis. The sizes of conventional and coated liposomes were found to be 104.6 ± 3.8 and 120.3 ± 6.4 nm with a low polydispersity index of 0.399 and 0.381, respectively. The ζ-potential of the liposomes was observed to be -16.56 mV, which changed to a positive value of +22.4 mV, clearly indicating the complete coating of the nanoliposomes by the chitosan. The average encapsulation efficiency was found to be between 70 and 80% for all prepared formulations. The compatibility of the drug with excipients and complete dispersion of the drug inside the system were verified by FTIR, XRD, and DSC studies. Furthermore, the in vitro release studies concluded the sustained release pattern following the Korsmeyer-Peppas model as the best-fitting model with Fickian diffusion. Ex vivo studies showed better permeation of the chitosan-coated liposomes, which was further confirmed by confocal studies. The prepared chitosan-coated liposomes showed superior antioxidant activity (94.56%) and enhanced % cytotoxicity (IC50 7.253 ± 0.34 μM) compared to the uncoated liposomes. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay displayed better cytotoxicity of the chitosan-coated nanoliposomes compared to the plain drug, showing the better penetration and enhanced bioavailability of drugs inside the cells. The formulation was found to be safe for administration, which was confirmed using the toxicity studies performed on an animal model. The above data suggested that poorly soluble lipophilic drugs could be successfully delivered via chitosan-coated liposomes for their effective delivery in breast cancer.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures











References
-
- Anderson B. O.; Ilbawi A. M.; Fidarova E.; Weiderpass E.; Stevens L.; Abdel-Wahab M.; Mikkelsen B. The Global Breast Cancer Initiative: A Strategic Collaboration to Strengthen Health Care for Non-Communicable Diseases. Lancet Oncol. 2021, 22 (5), 578–581. 10.1016/S1470-2045(21)00071-1. - DOI - PubMed
-
- Kawish S. M.; Hasan N.; Beg S.; Qadir A.; Jain G. K.; Aqil M.; Ahmad F. J. Docetaxel-Loaded Borage Seed Oil Nanoemulsion with Improved Antitumor Activity for Solid Tumor Treatment: Formulation Development, in Vitro, in Silico and in Vivo Evaluation. J. Drug Delivery Sci. Technol. 2022, 75, 103693 10.1016/j.jddst.2022.103693. - DOI
LinkOut - more resources
Full Text Sources
