Coupling of SARS-CoV-2 to Aβ Amyloid Fibrils
- PMID: 38434865
- PMCID: PMC10905702
- DOI: 10.1021/acsomega.3c08481
Coupling of SARS-CoV-2 to Aβ Amyloid Fibrils
Abstract
The COVID-19 infection has been more problematic for individuals with certain health predispositions. Coronaviruses could also interfere with neural diseases if the viruses succeed in entering the brain. Therefore, it might be of principal interest to examine a possible coupling of coronaviruses and amyloid fibrils. Here, molecular dynamics simulations were used to investigate direct coupling of SARS-CoV-2 and Aβ fibrils, which play a central role in neural diseases. The simulations revealed several stable binding configurations and their dynamics of Aβ42 fibrils attached to spike proteins of the Omicron and Alpha variants of SARS-CoV-2.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
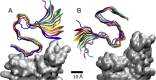

Similar articles
-
Comparative Analysis of Conformational Dynamics and Systematic Characterization of Cryptic Pockets in the SARS-CoV-2 Omicron BA.2, BA.2.75 and XBB.1 Spike Complexes with the ACE2 Host Receptor: Confluence of Binding and Structural Plasticity in Mediating Networks of Conserved Allosteric Sites.Viruses. 2023 Oct 10;15(10):2073. doi: 10.3390/v15102073. Viruses. 2023. PMID: 37896850 Free PMC article.
-
Computational Analysis of Short Linear Motifs in the Spike Protein of SARS-CoV-2 Variants Provides Possible Clues into the Immune Hijack and Evasion Mechanisms of Omicron Variant.Int J Mol Sci. 2022 Aug 8;23(15):8822. doi: 10.3390/ijms23158822. Int J Mol Sci. 2022. PMID: 35955954 Free PMC article.
-
Mechanistic insights into the mitigation of Aβ aggregation and protofibril destabilization by a D-enantiomeric decapeptide rk10.Phys Chem Chem Phys. 2022 Sep 21;24(36):21975-21994. doi: 10.1039/d2cp02601e. Phys Chem Chem Phys. 2022. PMID: 36069400
-
Resting microglia react to Aβ42 fibrils but do not detect oligomers or oligomer-induced neuronal damage.Neurobiol Aging. 2014 Nov;35(11):2444-2457. doi: 10.1016/j.neurobiolaging.2014.05.023. Epub 2014 May 29. Neurobiol Aging. 2014. PMID: 24973120
-
The evolution of the spike protein and hACE2 interface of SARS-CoV-2 omicron variants determined by hydrogen bond formation.Brief Funct Genomics. 2023 May 18;22(3):291-301. doi: 10.1093/bfgp/elac053. Brief Funct Genomics. 2023. PMID: 36723978
Cited by
-
SARS-CoV-2 induces Alzheimer's disease-related amyloid-β pathology in ex vivo human retinal explants and retinal organoids.Sci Adv. 2025 Jul 4;11(27):eads5006. doi: 10.1126/sciadv.ads5006. Epub 2025 Jul 4. Sci Adv. 2025. PMID: 40614201 Free PMC article.
-
Effect of a SARS-CoV-2 Protein Fragment on the Amyloidogenic Propensity of Human Islet Amyloid Polypeptide.ACS Chem Neurosci. 2024 Dec 18;15(24):4431-4440. doi: 10.1021/acschemneuro.4c00473. Epub 2024 Nov 24. ACS Chem Neurosci. 2024. PMID: 39582236 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
