Synthesis, Anticancer Activity, and In Silico Modeling of Alkylsulfonyl Benzimidazole Derivatives: Unveiling Potent Bcl-2 Inhibitors for Breast Cancer
- PMID: 38434899
- PMCID: PMC10905736
- DOI: 10.1021/acsomega.3c09411
Synthesis, Anticancer Activity, and In Silico Modeling of Alkylsulfonyl Benzimidazole Derivatives: Unveiling Potent Bcl-2 Inhibitors for Breast Cancer
Abstract
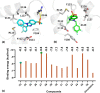
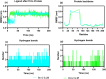
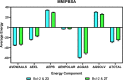
A series of alkylsulfonyl 1H-benzo[d]imidazole derivatives were synthesized and evaluated for anticancer activity against human breast cancer cells, MCF-7 in vitro. The cytotoxic potential was determined using the xCELLigence real-time cell analysis, and expression levels of genes related to microtubule organization, tumor suppression, apoptosis, cell cycle, and proliferation were examined by quantitative real-time polymerase chain reaction. Molecular docking against Bcl-2 was carried out using AutoDock Vina, while ADME studies were performed to predict the physicochemical and drug-likeness properties of the synthesized compounds. The results revealed that compounds 23 and 27 were the most potent cytotoxic derivatives against MCF-7 cells. Gene expression analysis showed that BCL-2 was the most prominent gene studied. Treatment of MCF-7 cells with compounds 23 and 27 resulted in significant downregulation of the BCL-2 gene, with fold changes of 128 and 256, respectively. Docking analysis predicted a strong interaction between the compounds and the target protein. Interestingly, all of the compounds exhibit a higher binding affinity toward Bcl-2 than the standard drug (compound 27 vina score = -9.6 kcal/mol, vincristine = -6.7 kcal/mol). Molecular dynamics simulations of compounds 23 and 27 showed a permanent stabilization in the binding site of Bcl-2 for 200 ns. Based on Lipinski and Veber's filters, all synthesized compounds displayed drug-like characteristics. These findings suggest that compounds 23 and 27 were the most promising cytotoxic compounds and downregulated the expression of the BCL-2 gene. These derivatives could be further explored as potential candidates for the treatment of breast cancer.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


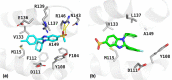


References
LinkOut - more resources
Full Text Sources
Miscellaneous
