Theoretical Exploration of Enhanced Antioxidant Activity in Copper Complexes of Tetrahydroxystilbenes: Insights into Mechanisms and Molecular Interactions
- PMID: 38434904
- PMCID: PMC10906065
- DOI: 10.1021/acsomega.3c07885
Theoretical Exploration of Enhanced Antioxidant Activity in Copper Complexes of Tetrahydroxystilbenes: Insights into Mechanisms and Molecular Interactions
Abstract
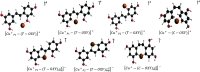
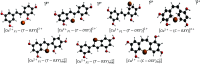
A theoretical investigation was conducted using DFT/PW91/TZP/DMSO calculations on a complete set of exhaustive lists of 18 compounds resulting from the complexation of trans-2,4,3',5'-tetrahydroxystilbene (T-OXY) and cis-2,4,1',3'-tetrahydroxystilbene (C-OXY) with copper metal cations (Cu+ and Cu2+). The ligand-binding sites are the critical points of Quantum Theory of Atoms in Molecules (QTAIM) analysis on neutral and deprotonated ligands. Various mechanisms, including hydrogen atom transfer (HAT), sequential proton loss electron transfer (SPLET), single electron transfer followed by proton transfer (SET-PT), and bond dissociation energy (BDE(E0)) calculations, were employed to quantify the antioxidant activity. The BDE(E0) mechanism emerged as the most suitable approach for such analyses to evaluate the departure of hydrogen atoms since the results show the HAT mechanism is the most likely occurring. Particularly intriguing were the anionic Cu+ complexes with ligands adopting trans configurations and deprotonated conformations, displaying superior antioxidant activity compared to their counterparts. Remarkably, a single ligand within the Cu+ complex exhibited exceptional antioxidant prowess, yielding a BDE(E0) value of 91.47 kcal/mol. Furthermore, a complex involving two deprotonated ligands demonstrated antioxidant activity of 31.12 kcal/mol, signifying its potential as a potent antiradical agent, surpassing T-OXY by a factor of 3.91 and even surpassing the antioxidant efficiency of Vitamin C.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
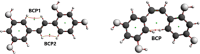
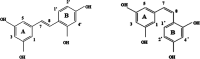





Similar articles
-
Theoretical Design of New Grafted Molecules d-Glucosamine-Oxyresveratrol-Essential Amino Acids: DFT Evaluation of the Structure-Antioxidant Activity.ACS Omega. 2024 Aug 20;9(35):37128-37140. doi: 10.1021/acsomega.4c04356. eCollection 2024 Sep 3. ACS Omega. 2024. PMID: 39246505 Free PMC article.
-
DFT and QTAIM based investigation on the structure and antioxidant behavior of lichen substances Atranorin, Evernic acid and Diffractaic acid.Comput Biol Chem. 2019 Jun;80:66-78. doi: 10.1016/j.compbiolchem.2019.03.009. Epub 2019 Mar 23. Comput Biol Chem. 2019. PMID: 30928870
-
DFT studies of trans and cis influences in the homolysis of the Co-C bond in models of the alkylcobalamins.J Phys Chem A. 2013 Apr 11;117(14):3057-68. doi: 10.1021/jp311788t. Epub 2013 Apr 2. J Phys Chem A. 2013. PMID: 23510290
-
Recent advances in the antioxidant activity and mechanisms of chalcone derivatives: a computational review.Free Radic Res. 2022 May-Jun;56(5-6):378-397. doi: 10.1080/10715762.2022.2120396. Epub 2022 Sep 8. Free Radic Res. 2022. PMID: 36063087 Review.
-
[CuO](+) and [CuOH](2+) complexes: intermediates in oxidation catalysis?Acc Chem Res. 2015 Jul 21;48(7):2126-31. doi: 10.1021/acs.accounts.5b00169. Epub 2015 Jun 15. Acc Chem Res. 2015. PMID: 26075312 Free PMC article. Review.
Cited by
-
Synthesis, Characterization and Assessment of Antioxidant and Melanogenic Inhibitory Properties of Edaravone Derivatives.Antioxidants (Basel). 2024 Sep 23;13(9):1148. doi: 10.3390/antiox13091148. Antioxidants (Basel). 2024. PMID: 39334807 Free PMC article.
-
Theoretical Design of New Grafted Molecules d-Glucosamine-Oxyresveratrol-Essential Amino Acids: DFT Evaluation of the Structure-Antioxidant Activity.ACS Omega. 2024 Aug 20;9(35):37128-37140. doi: 10.1021/acsomega.4c04356. eCollection 2024 Sep 3. ACS Omega. 2024. PMID: 39246505 Free PMC article.
References
LinkOut - more resources
Full Text Sources
