Platinum-Nickel Nanowires with Improved Hydrogen Evolution Performance in Anion Exchange Membrane-Based Electrolysis
- PMID: 38435051
- PMCID: PMC10906943
- DOI: 10.1021/acscatal.0c01568
Platinum-Nickel Nanowires with Improved Hydrogen Evolution Performance in Anion Exchange Membrane-Based Electrolysis
Abstract
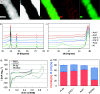
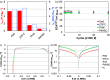
Platinum-nickel (Pt-Ni) nanowires were developed as hydrogen evolving catalysts for anion exchange membrane electrolyzers. Following synthesis by galvanic displacement, the nanowires had Pt surface areas of 90 m2 gPt-1. The nanowire specific exchange current densities were 2-3 times greater than commercial nanoparticles and may benefit from the extended nanostructure morphology that avoids fringe facets and produces higher quantities of Pt{100}. Hydrogen annealing was used to alloy Pt and Ni zones and compress the Pt lattice. Following annealing, the nanowire activity improved to 4 times greater than the as-synthesized wires and 10 times greater than Pt nanoparticles. Density functional theory calculations were performed to investigate the influence of lattice compression and exposed facet on the water-splitting reaction; it was found that at a lattice of 3.77 Å, the (100) facet of a Pt-skin grown on Ni3Pt weakens hydrogen binding and lowers the barrier to water-splitting as compared to pure Pt(100). Moreover, the activation energy of water-splitting on the (100) facet of a Pt-skin grown on Ni3Pt is particularly advantageous at 0.66 eV as compared to the considerably higher 0.90 eV required on (111) surfaces of pure Pt or Pt-skin grown on Ni3Pt. This favorable effect may be slightly mitigated during further optimization procedures such as acid leaching near-surface Ni, necessary to incorporate the nanowires into electrolyzer membrane electrode assemblies. Exposure to acid resulted in slight dealloying and Pt lattice expansion, which reduced half-cell activity, but exposed Pt surfaces and improved single-cell performance. Membrane electrode assembly performance was kinetically 1-2 orders of magnitude greater than Ni and slightly better than Pt nanoparticles while at one tenth the Pt loading. These electrocatalysts potentially exploit the highly active {100} facets and provide an ultralow Pt group metal option that can enable anion exchange membrane electrolysis, bridging the gap to proton exchange membrane-based systems.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Pivovar B.H2 at Scale, NREL Workshop; Department of Energy, U. S., https://www.energy.gov/sites/prod/files/2016/12/f34/fcto_h2atscale_works..., 2016.confprof
-
- Pivovar B.; Rustagi N.; Satyapal S. Hydrogen at Scale (H2@ Scale): Key to a Clean, Economic, and Sustainable Energy System. Electrochem. Soc. Interface 2018, 27, 47–52. 10.1149/2.f04181if. - DOI
-
- Alia S. M.H2@Scale: Experimental Characterization of Durability of Advanced Electrolyzer Concepts in Dynamic Loading; Department of Energy, U. S., https://www.hydrogen.energy.gov/pdfs/review19/ta022_alia_2019_o.pdf, 2019.
-
- Pourbaix M.Atlas of Electrochemical Equilibria in Aqueous Solutions, 2nd ed.; National Association of Corrosion Engineers: Houston, Texas, 1974.
-
- Pavel C. C.; Cecconi F.; Emiliani C.; Santiccioli S.; Scaffidi A.; Catanorchi S.; Comotti M. Highly Efficient Platinum Group Metal Free Based Membrane-Electrode Assembly for Anion Exchange Membrane Water Electrolysis. Angew. Chem., Int. Ed. 2014, 53, 1378–1381. 10.1002/anie.201308099. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
