Vaccination for Patients Receiving Dialysis
- PMID: 38435066
- PMCID: PMC10906410
- DOI: 10.1016/j.xkme.2023.100775
Vaccination for Patients Receiving Dialysis
Abstract
Vaccinating patients receiving dialysis may prevent morbidity and mortality in this vulnerable population. The National Forum of End-Stage Renal Disease Networks (the Forum) published a revised vaccination toolkit in 2021 to update evidence and recommendations on vaccination for patients receiving dialysis. Significant changes in the last 10 years include more data supporting the use of a high-dose influenza vaccine, the introduction of the Heplisav-B vaccine for hepatitis B, and changes in pneumococcal vaccines, including the approval of the PCV15 and PCV20 to replace the PCV13 and PPSV23 vaccines. Additional key items include the introduction of vaccines against severe acute respiratory syndrome coronavirus 2, the virus that causes coronavirus disease 2019 (COVID-19), and a new vaccine to prevent respiratory syncytial virus disease. Historically, influenza and pneumococcal vaccinations were routinely administered by dialysis facilities, and because of possible risks of hematogenous spread of hepatitis B, dialysis providers often have detailed hepatitis B vaccine protocols. In March 2021, COVID-19 vaccines were made available for dialysis facilities to administer, although with the end of the public health emergency, vaccine policies by dialysis facilities against COVID-19 remains uncertain. The respiratory syncytial virus vaccine was authorized in 2023, and how dialysis facilities will approach this vaccine also remains uncertain. This review summarizes the Forum's vaccination toolkit and discusses the role of the dialysis facility in vaccinating patients to reduce the risk of severe infections.
Keywords: Dialysis; SARS-CoV2; influenza; pneumococcus; vaccination.
© 2023 The Authors.
Figures
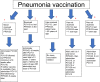
1. Prevnar 15 or 20 vaccine is only given once in lifetime of the patient.
2. PPSV23 vaccine can be given as many as 3 times in the life of the patient.
3. One dose of Prevnar 20 will complete a patient’s pneumonia vaccination.
4. One dose of Prevnar 15 will require one additional dose of PPSV23 to complete vaccination series.
5. Prevnar 15 or 20 vaccine cannot be given up to a year after receiving PPSV23 vaccine.
6. PPSV23 can be given as early as 8 weeks after giving Prevnar 15.
7. PPSV23 is readministered 5 years after giving first dose in patients less than 65 year of age and then again after age of 65, assuming at least 5 years elapsed since the last dose.
8. After age 65, only 1 dose of PPSV23 is necessary.
References
-
- Murthy N., Wodi A.P., Bernstein H., Ault K.A. Advisory Committee on Immunization Practices†, Advisory Committee on Immunization Practices. Recommended adult immunization schedule, United States, 2022. Ann Intern Med. 2022;175(3):432–443. - PubMed
-
- Sam R., Henner D., Kalantar-Zadeh K., et al. Vaccination toolkit. https://esrdnetworks.org/toolkits/professional-toolkits/vaccination-tool...
-
- Cohen-Hagai K., Kotliroff A., Rozenberg I., Korzets Z., Zitman-Gal T., Benchetrit S. Effectiveness of influenza vaccine in hemodialysis patients: A retrospective study. Ther Apher Dial. 2019;23(1):38–43. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

