Assessing the clinical benefit, safety, and patient-reported outcomes with the use of the PAHcare™ digital platform in pulmonary arterial hypertension: a pilot study
- PMID: 38435295
- PMCID: PMC10904626
- DOI: 10.3389/fpubh.2024.1335072
Assessing the clinical benefit, safety, and patient-reported outcomes with the use of the PAHcare™ digital platform in pulmonary arterial hypertension: a pilot study
Abstract
Introduction: Digital health interventions, particularly mobile health platforms, have shown promise in supporting patients with respiratory conditions, but their application in pulmonary arterial hypertension (PAH) remains limited. We aimed to assess the feasibility, acceptability, and potential clinical benefit of the novel PAHcare™ digital platform as a patient-centred intervention for PAH management through a prospective, single-arm, multicenter pilot study conducted on 53 patients diagnosed with PAH who used the platform for 6 months.
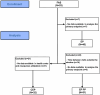
Methods: The primary objective was to assess the impact on Health-Related Quality of Life (HRQoL) through questionnaires. Secondary objectives included evaluating clinical outcomes, including disease progression, PAH signs and symptoms, the 6-min walking test, and the patient's symptom perception. Additionally, we assessed patient satisfaction and engagement with the PAHcare™ platform, interaction with health coaches, retention, costs and healthcare resource utilisation (HCRU), and safety through monitoring device incidents.
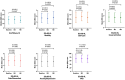
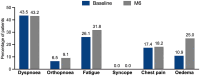
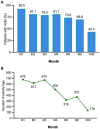
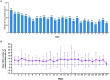
Results: Minimal changes in HRQoL and clinical outcomes were observed over 6 months. A noteworthy 92.4% of patients actively used the platform in the first month, maintaining high usage throughout the study. Patient satisfaction was substantial, with more than half of the patients expressing excellence in service quality, willingness to reuse the platform, and fulfilment of their needs. Health coach interaction was high, with 76% of patients initiating contact within the first week. User retention rates were 70%, with prevalent ongoing usage and interaction with healthcare professionals even after the study. In terms of HCRU and costs, the study showed no significant changes in PAH-related hospital admissions, clinical visits, or tests. Finally, the low number of device-related incidents indicated platform safety.
Conclusion: This pilot study provides compelling evidence supporting the feasibility and acceptability of the PAHcare™ digital platform to empower patients to manage their disease and significantly enhance their overall experience with PAH.
Keywords: digital intervention platform; electronic patient-reported outcome; health services research; mobile health (mHealth); patient support program; pulmonary arterial hypertension.
Copyright © 2024 Peñate, Parra, Morera, Meñaca, Ramón, Menéndez, Marrero, de la Cal, Ghadban-Garrido, Tolosana, Puentes, Aguayo, Mahdavi, Jeanneret and Subías.
Conflict of interest statement
PE has received consulting and lecture fees by Ferrer Internacional S.A. (Barcelona, Spain), Gossamer Bio., AOP Health, Janssen, and MSD. GP has received consulting fees by Ferrer Internacional S.A. (Barcelona, Spain). RA, HM, and GB are employees of Ferrer Internacional (Barcelona, Spain). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

