Dysbiosis of the gut microbiota and its effect on α-synuclein and prion protein misfolding: consequences for neurodegeneration
- PMID: 38435303
- PMCID: PMC10904658
- DOI: 10.3389/fcimb.2024.1348279
Dysbiosis of the gut microbiota and its effect on α-synuclein and prion protein misfolding: consequences for neurodegeneration
Abstract
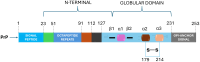
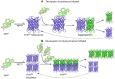
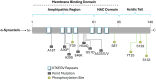
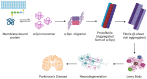
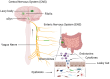
Abnormal behavior of α-synuclein and prion proteins is the hallmark of Parkinson's disease (PD) and prion illnesses, respectively, being complex neurological disorders. A primary cause of protein aggregation, brain injury, and cognitive loss in prion illnesses is the misfolding of normal cellular prion proteins (PrPC) into an infectious form (PrPSc). Aggregation of α-synuclein causes disruptions in cellular processes in Parkinson's disease (PD), leading to loss of dopamine-producing neurons and motor symptoms. Alteration in the composition or activity of gut microbes may weaken the intestinal barrier and make it possible for prions to go from the gut to the brain. The gut-brain axis is linked to neuroinflammation; the metabolites produced by the gut microbiota affect the aggregation of α-synuclein, regulate inflammation and immunological responses, and may influence the course of the disease and neurotoxicity of proteins, even if their primary targets are distinct proteins. This thorough analysis explores the complex interactions that exist between the gut microbiota and neurodegenerative illnesses, particularly Parkinson's disease (PD) and prion disorders. The involvement of the gut microbiota, a complex collection of bacteria, archaea, fungi, viruses etc., in various neurological illnesses is becoming increasingly recognized. The gut microbiome influences neuroinflammation, neurotransmitter synthesis, mitochondrial function, and intestinal barrier integrity through the gut-brain axis, which contributes to the development and progression of disease. The review delves into the molecular mechanisms that underlie these relationships, emphasizing the effects of microbial metabolites such as bacterial lipopolysaccharides (LPS), and short-chain fatty acids (SCFAs) in regulating brain functioning. Additionally, it looks at how environmental influences and dietary decisions affect the gut microbiome and whether they could be risk factors for neurodegenerative illnesses. This study concludes by highlighting the critical role that the gut microbiota plays in the development of Parkinson's disease (PD) and prion disease. It also provides a promising direction for future research and possible treatment approaches. People afflicted by these difficult ailments may find hope in new preventive and therapeutic approaches if the role of the gut microbiota in these diseases is better understood.
Keywords: Parkinson’s disease; and neurodegeneration; gut microbiota; neuro-inflammation; prion disease; prion protein; short-chain fatty acids; α-synuclein.
Copyright © 2024 Mahbub, Islam, Hong and Chung.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Parkinson's disease and the gut microbiota connection: unveiling dysbiosis and exploring therapeutic horizons.Neuroscience. 2025 Aug 16;581:1-15. doi: 10.1016/j.neuroscience.2025.07.003. Epub 2025 Jul 2. Neuroscience. 2025. PMID: 40614920 Review.
-
Gut-Brain Axis a Key Player to Control Gut Dysbiosis in Neurological Diseases.Mol Neurobiol. 2024 Dec;61(12):9873-9891. doi: 10.1007/s12035-023-03691-3. Epub 2023 Oct 18. Mol Neurobiol. 2024. PMID: 37851313 Review.
-
Disease mechanisms as subtypes: Microbiome.Handb Clin Neurol. 2023;193:107-131. doi: 10.1016/B978-0-323-85555-6.00006-0. Handb Clin Neurol. 2023. PMID: 36803806 Review.
-
Gut microbiome, short-chain fatty acids, alpha-synuclein, neuroinflammation, and ROS/RNS: Relevance to Parkinson's disease and therapeutic implications.Redox Biol. 2024 May;71:103092. doi: 10.1016/j.redox.2024.103092. Epub 2024 Feb 16. Redox Biol. 2024. PMID: 38377788 Free PMC article. Review.
-
The gut microbiome in Parkinson's disease: A culprit or a bystander?Prog Brain Res. 2020;252:357-450. doi: 10.1016/bs.pbr.2020.01.004. Epub 2020 Mar 5. Prog Brain Res. 2020. PMID: 32247371 Review.
Cited by
-
The Biological Activity of Ganoderma lucidum on Neurodegenerative Diseases: The Interplay between Different Active Compounds and the Pathological Hallmarks.Molecules. 2024 May 26;29(11):2516. doi: 10.3390/molecules29112516. Molecules. 2024. PMID: 38893392 Free PMC article. Review.
-
Probiotics as Potential Treatments for Neurodegenerative Diseases: a Review of the Evidence from in vivo to Clinical Trial.Biomol Ther (Seoul). 2025 Jan 1;33(1):54-74. doi: 10.4062/biomolther.2024.215. Epub 2024 Dec 16. Biomol Ther (Seoul). 2025. PMID: 39676295 Free PMC article. Review.
-
Decoding Neurodegeneration: A Review of Molecular Mechanisms and Therapeutic Advances in Alzheimer's, Parkinson's, and ALS.Int J Mol Sci. 2024 Nov 24;25(23):12613. doi: 10.3390/ijms252312613. Int J Mol Sci. 2024. PMID: 39684324 Free PMC article. Review.
-
Longitudinal microbiome investigation throughout prion disease course reveals pre- and symptomatic compositional perturbations linked to short-chain fatty acid metabolism and cognitive impairment in mice.Front Microbiol. 2024 Jun 11;15:1412765. doi: 10.3389/fmicb.2024.1412765. eCollection 2024. Front Microbiol. 2024. PMID: 38919500 Free PMC article.
-
Alpha Synuclein Toxicity and Non-Motor Parkinson's.Cells. 2024 Jul 27;13(15):1265. doi: 10.3390/cells13151265. Cells. 2024. PMID: 39120295 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

