Pioneering PGC-1α-boosted secretome: a novel approach to combating liver fibrosis
- PMID: 38435492
- PMCID: PMC10902621
- DOI: 10.4174/astr.2024.106.3.155
Pioneering PGC-1α-boosted secretome: a novel approach to combating liver fibrosis
Abstract
Purpose: Liver fibrosis is a critical health issue with limited treatment options. This study investigates the potential of PGC-Sec, a secretome derived from peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α)-overexpressing adipose-derived stem cells (ASCs), as a novel therapeutic strategy for liver fibrosis.
Methods: Upon achieving a cellular confluence of 70%-80%, ASCs were transfected with pcDNA-PGC-1α. PGC-Sec, obtained through concentration of conditioned media using ultrafiltration units with a 3-kDa cutoff, was assessed through in vitro assays and in vitro mouse models.
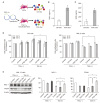
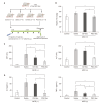
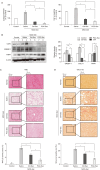
Results: In vitro, PGC-Sec significantly reduced LX2 human hepatic stellate cell proliferation and mitigated mitochondrial oxidative stress compared to the control-secretome. In an in vivo mouse model, PGC-Sec treatment led to notable reductions in hepatic enzyme activity, serum proinflammatory cytokine concentrations, and fibrosis-related marker expression. Histological analysis demonstrated improved liver histology and reduced fibrosis severity in PGC-Sec-treated mice. Immunohistochemical staining confirmed enhanced expression of PGC-1α, optic atrophy 1 (a mitochondrial function marker), and peroxisome proliferator-activated receptor alpha (an antifibrogenic marker) in the PGC-Sec-treated group, along with reduced collagen type 1A expression (a profibrogenic marker).
Conclusion: These findings highlight the therapeutic potential of PGC-Sec in combating liver fibrosis by enhancing mitochondrial biogenesis and function, and promoting antifibrotic processes. PGC-Sec holds promise as a novel treatment strategy for liver fibrosis.
Keywords: Adipose-derived stem cells; Liver fibrosis; Peroxisome proliferator-activated receptor-gamma coactivator-1 alpha; Secretome.
Copyright © 2024, the Korean Surgical Society.
Conflict of interest statement
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Figures


References
-
- Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14:181–194. - PubMed
-
- Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35:851–858. - PubMed
-
- Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21:363–383. - PubMed
LinkOut - more resources
Full Text Sources

