Hydrogen-Deuterium Exchange Mass Spectrometry Identifies Local and Long-Distance Interactions within the Multicomponent Radical SAM Enzyme, PqqE
- PMID: 38435514
- PMCID: PMC10906245
- DOI: 10.1021/acscentsci.3c01023
Hydrogen-Deuterium Exchange Mass Spectrometry Identifies Local and Long-Distance Interactions within the Multicomponent Radical SAM Enzyme, PqqE
Abstract
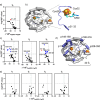
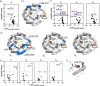
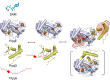
Interactions among proteins and peptides are essential for many biological activities including the tailoring of peptide substrates to produce natural products. The first step in the production of the bacterial redox cofactor pyrroloquinoline quinone (PQQ) from its peptide precursor is catalyzed by a radical SAM (rSAM) enzyme, PqqE. We describe the use of hydrogen-deuterium exchange mass spectrometry (HDX-MS) to characterize the structure and conformational dynamics in the protein-protein and protein-peptide complexes necessary for PqqE function. HDX-MS-identified hotspots can be discerned in binary and ternary complex structures composed of the peptide PqqA, the peptide-binding chaperone PqqD, and PqqE. Structural conclusions are supported by size-exclusion chromatography coupled to small-angle X-ray scattering (SEC-SAXS). HDX-MS further identifies reciprocal changes upon the binding of substrate peptide and S-adenosylmethionine (SAM) to the PqqE/PqqD complex: long-range conformational alterations have been detected upon the formation of a quaternary complex composed of PqqA/PqqD/PqqE and SAM, spanning nearly 40 Å, from the PqqA binding site in PqqD to the PqqE active site Fe4S4. Interactions among the various regions are concluded to arise from both direct contact and distal communication. The described experimental approach can be readily applied to the investigation of protein conformational communication among a large family of peptide-modifying rSAM enzymes.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
PqqD is a novel peptide chaperone that forms a ternary complex with the radical S-adenosylmethionine protein PqqE in the pyrroloquinoline quinone biosynthetic pathway.J Biol Chem. 2015 May 15;290(20):12908-18. doi: 10.1074/jbc.M115.646521. Epub 2015 Mar 27. J Biol Chem. 2015. PMID: 25817994 Free PMC article.
-
Demonstration That the Radical S-Adenosylmethionine (SAM) Enzyme PqqE Catalyzes de Novo Carbon-Carbon Cross-linking within a Peptide Substrate PqqA in the Presence of the Peptide Chaperone PqqD.J Biol Chem. 2016 Apr 22;291(17):8877-84. doi: 10.1074/jbc.C115.699918. Epub 2016 Mar 8. J Biol Chem. 2016. PMID: 26961875 Free PMC article.
-
(1)H, (13)C, and (15)N resonance assignments and secondary structure information for Methylobacterium extorquens PqqD and the complex of PqqD with PqqA.Biomol NMR Assign. 2016 Oct;10(2):385-9. doi: 10.1007/s12104-016-9705-8. Epub 2016 Sep 16. Biomol NMR Assign. 2016. PMID: 27638737 Free PMC article.
-
Biogenesis of the peptide-derived redox cofactor pyrroloquinoline quinone.Curr Opin Chem Biol. 2020 Dec;59:93-103. doi: 10.1016/j.cbpa.2020.05.001. Epub 2020 Jul 27. Curr Opin Chem Biol. 2020. PMID: 32731194 Free PMC article. Review.
-
Measuring the hydrogen/deuterium exchange of proteins at high spatial resolution by mass spectrometry: overcoming gas-phase hydrogen/deuterium scrambling.Acc Chem Res. 2014 Oct 21;47(10):3018-27. doi: 10.1021/ar500194w. Epub 2014 Aug 29. Acc Chem Res. 2014. PMID: 25171396 Review.
Cited by
-
Dynamically Interacting Protein Networks Provide a Mechanism to Overcome the Enormous Intrinsic Barrier to Orotidine 5'-Monophosphate Decarboxylation.ACS Cent Sci. 2025 Jul 11;11(8):1377-1390. doi: 10.1021/acscentsci.5c00590. eCollection 2025 Aug 27. ACS Cent Sci. 2025. PMID: 40893966 Free PMC article.
-
Aromatic side-chain crosslinking in RiPP biosynthesis.Nat Chem Biol. 2025 Feb;21(2):168-181. doi: 10.1038/s41589-024-01795-y. Epub 2025 Jan 15. Nat Chem Biol. 2025. PMID: 39814993 Free PMC article. Review.
-
Identification of Scaffold Specific Energy Transfer Networks in the Enthalpic Activation of Orotidine 5'-Monophosphate Decarboxylase.bioRxiv [Preprint]. 2025 Jan 29:2025.01.29.635545. doi: 10.1101/2025.01.29.635545. bioRxiv. 2025. PMID: 39975186 Free PMC article. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources
