Stereoconvergent and Chemoenzymatic Synthesis of Tumor-Associated Glycolipid Disialosyl Globopentaosylceramide for Probing the Binding Affinity of Siglec-7
- PMID: 38435515
- PMCID: PMC10906248
- DOI: 10.1021/acscentsci.3c01170
Stereoconvergent and Chemoenzymatic Synthesis of Tumor-Associated Glycolipid Disialosyl Globopentaosylceramide for Probing the Binding Affinity of Siglec-7
Abstract
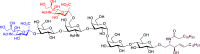
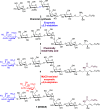
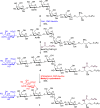
Disialosyl globopentaosylceramide (DSGb5) is a tumor-associated complex glycosphingolipid. However, the accessibility of structurally well-defined DSGb5 for precise biological functional studies remains challenging. Herein, we describe the first total synthesis of DSGb5 glycolipid by an efficient chemoenzymatic approach. A Gb5 pentasaccharide-sphingosine was chemically synthesized by a convergent and stereocontrolled [2 + 3] method using an oxazoline disaccharide donor to exclusively form β-anomeric linkage. After investigating the substrate specificity of different sialyltransferases, regio- and stereoselective installment of two sialic acids was achieved by two sequential enzyme-catalyzed reactions using α2,3-sialyltransferase Cst-I and α2,6-sialyltransferase ST6GalNAc5. A unique aspect of the approach is that methyl-β-cyclodextrin-assisted enzymatic α2,6-sialylation of glycolipid substrate enables installment of the challenging internal α2,6-linked sialoside to synthesize DSGb5 glycosphingolipid. Surface plasmon resonance studies indicate that DSGb5 glycolipid exhibits better binding affinity for Siglec-7 than the oligosaccharide moiety of DSGb5. The binding results suggest that the ceramide moiety of DSGb5 facilitates its binding by presenting multivalent interactions of glycan epitope for the recognition of Siglec-7.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
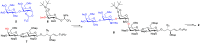




Similar articles
-
Chemoenzymatic synthesis of the oligosaccharide moiety of the tumor-associated antigen disialosyl globopentaosylceramide.Org Biomol Chem. 2019 Aug 7;17(31):7304-7308. doi: 10.1039/c9ob01368g. Org Biomol Chem. 2019. PMID: 31339142 Free PMC article.
-
A Modular Chemoenzymatic Synthesis of Disialosyl Globopentaosylceramide (DSGb5Cer) Glycan.J Org Chem. 2020 Dec 18;85(24):15920-15935. doi: 10.1021/acs.joc.0c01091. Epub 2020 Jul 5. J Org Chem. 2020. PMID: 32567311
-
Chemoenzymatic Synthesis of DSGb5 and Sialylated Globo-series Glycans.Angew Chem Int Ed Engl. 2019 Aug 12;58(33):11273-11278. doi: 10.1002/anie.201903943. Epub 2019 Jul 4. Angew Chem Int Ed Engl. 2019. PMID: 31140679
-
The sialyl-alpha2,6-lactosaminyl-structure: biosynthesis and functional role.Glycoconj J. 2000 Oct;17(10):669-76. doi: 10.1023/a:1011077000164. Glycoconj J. 2000. PMID: 11425186 Review.
-
Advanced Chemical Methods for Stereoselective Sialylation and Their Applications in Sialoglycan Syntheses.Chem Rec. 2021 Nov;21(11):3194-3223. doi: 10.1002/tcr.202100080. Epub 2021 May 24. Chem Rec. 2021. PMID: 34028159 Review.
Cited by
-
Organic Synthesis and Catalysis Enable Facile Access to Bioactive Compounds and Drugs.ACS Cent Sci. 2024 Dec 16;11(1):1-5. doi: 10.1021/acscentsci.4c02041. eCollection 2025 Jan 22. ACS Cent Sci. 2024. PMID: 39866703 Free PMC article. No abstract available.
-
Stereospecific Synthesis and Biological Evaluation of KRN7000 Analogues with Thio-modifications at the Acyl Moiety.ACS Med Chem Lett. 2024 Jun 5;15(7):1102-1108. doi: 10.1021/acsmedchemlett.4c00199. eCollection 2024 Jul 11. ACS Med Chem Lett. 2024. PMID: 39015265 Free PMC article.
-
Sugar Auxiliary Group Assisted Diversity-Oriented Enzymatic Modular Synthesis of 0-Series Ganglioside Glycans.Angew Chem Int Ed Engl. 2025 Feb 10;64(7):e202418929. doi: 10.1002/anie.202418929. Epub 2025 Jan 7. Angew Chem Int Ed Engl. 2025. PMID: 39714328
-
Insights into Siglec-7 Binding to Gangliosides: NMR Protein Assignment and the Impact of Ligand Flexibility.Adv Sci (Weinh). 2025 Jun;12(21):e2415782. doi: 10.1002/advs.202415782. Epub 2025 Apr 26. Adv Sci (Weinh). 2025. PMID: 40285643 Free PMC article.
-
Glycosphingolipids: from metabolism to chemoenzymatic total synthesis.Org Biomol Chem. 2024 Aug 22;22(33):6665-6683. doi: 10.1039/d4ob00695j. Org Biomol Chem. 2024. PMID: 39120686 Free PMC article. Review.
References
-
- Nguyen L.; McCord K. A.; Bui D. T.; Bouwman K. M.; Kitova E. N.; Elaish M.; Kumawat D.; Daskhan G. C.; Tomris I.; Han L.; Chopra P.; Yang T.-J.; Willows S. D.; Mason A. L.; Mahal L. K.; Lowary T. L.; West L. J.; Hsu S.-T. D.; Hobman T.; Tompkins S. M.; Boons G.-J.; de Vries R. P.; Macauley M. S.; Klassen J. S. Sialic acid-containing glycolipids mediate binding and viral entry of SARS-CoV-2. Nat. Chem. Biol. 2022, 18, 81–90. 10.1038/s41589-021-00924-1. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
