High-Performance Genetically Encoded Green Fluorescent Biosensors for Intracellular l-Lactate
- PMID: 38435524
- PMCID: PMC10906044
- DOI: 10.1021/acscentsci.3c01250
High-Performance Genetically Encoded Green Fluorescent Biosensors for Intracellular l-Lactate
Abstract
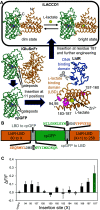
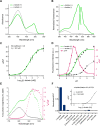
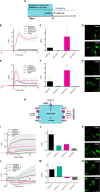
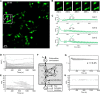
l-Lactate is a monocarboxylate produced during the process of cellular glycolysis and has long generally been considered a waste product. However, studies in recent decades have provided new perspectives on the physiological roles of l-lactate as a major energy substrate and a signaling molecule. To enable further investigations of the physiological roles of l-lactate, we have developed a series of high-performance (ΔF/F = 15 to 30 in vitro), intensiometric, genetically encoded green fluorescent protein (GFP)-based intracellular l-lactate biosensors with a range of affinities. We evaluated these biosensors in cultured cells and demonstrated their application in an ex vivo preparation of Drosophila brain tissue. Using these biosensors, we were able to detect glycolytic oscillations, which we analyzed and mathematically modeled.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Hill A. V.; Kupalov P. Anaerobic and Aerobic Activity in Isolated Muscle. Proc. R. Soc. London B Biol. Sci. 1929, 105 (737), 313–322. 10.1098/rspb.1929.0045. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
