Metals in Cancer Research: Beyond Platinum Metallodrugs
- PMID: 38435529
- PMCID: PMC10906246
- DOI: 10.1021/acscentsci.3c01340
Metals in Cancer Research: Beyond Platinum Metallodrugs
Abstract
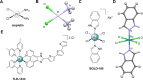
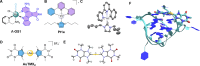
The discovery of the medicinal properties of platinum complexes has fueled the design and synthesis of new anticancer metallodrugs endowed with unique modes of action (MoA). Among the various families of experimental antiproliferative agents, organometallics have emerged as ideal platforms to control the compounds' reactivity and stability in a physiological environment. This is advantageous to efficiently deliver novel prodrug activation strategies, as well as to design metallodrugs acting only via noncovalent interactions with their pharmacological targets. Noteworthy, another justification for the advance of organometallic compounds for therapy stems from their ability to catalyze bioorthogonal reactions in cancer cells. When not yet ideal as drug leads, such compounds can be used as selective chemical tools that benefit from the advantages of catalytic amplification to either label the target of interest (e.g., proteins) or boost the output of biochemical signals. Examples of metallodrugs for the so-called "catalysis in cells" are considered in this Outlook together with other organometallic drug candidates. The selected case studies are discussed in the frame of more general challenges in the field of medicinal inorganic chemistry.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Intracellular Catalysis with Selected Metal Complexes and Metallic Nanoparticles: Advances toward the Development of Catalytic Metallodrugs.Chem Rev. 2019 Jan 23;119(2):829-869. doi: 10.1021/acs.chemrev.8b00493. Epub 2019 Jan 8. Chem Rev. 2019. PMID: 30618246 Review.
-
Novel metals and metal complexes as platforms for cancer therapy.Curr Pharm Des. 2010 Jun;16(16):1813-25. doi: 10.2174/138161210791209009. Curr Pharm Des. 2010. PMID: 20337575 Free PMC article. Review.
-
Catalysis Concepts in Medicinal Inorganic Chemistry.Chemistry. 2019 May 10;25(27):6651-6660. doi: 10.1002/chem.201806341. Epub 2019 Feb 25. Chemistry. 2019. PMID: 30681213
-
Next-generation anticancer metallodrugs.Curr Top Med Chem. 2012;12(3):219-35. doi: 10.2174/156802612799078964. Curr Top Med Chem. 2012. PMID: 22236158 Review.
-
Cyclometalated Complexes of Platinum and Gold with Biological Properties: State-of-the-Art and Future Perspectives.Curr Med Chem. 2018 Feb 12;25(4):437-461. doi: 10.2174/0929867324666170529125229. Curr Med Chem. 2018. PMID: 28554319 Review.
Cited by
-
Coordinative Compounds Based on Unsaturated Carboxylate with Versatile Biological Applications.Molecules. 2024 May 15;29(10):2321. doi: 10.3390/molecules29102321. Molecules. 2024. PMID: 38792182 Free PMC article. Review.
-
Ligand supplementation restores the cancer therapy efficacy of the antirheumatic drug auranofin from serum inactivation.Nat Commun. 2025 Aug 9;16(1):7347. doi: 10.1038/s41467-025-62634-9. Nat Commun. 2025. PMID: 40783561 Free PMC article.
-
(η6-Benzene)-chlorido-[2-(pyridin-2-yl)quinoline-κ2 N,N']ruthenium(II) tetra-fluorido-borate.IUCrdata. 2025 Jan 7;10(Pt 1):x241240. doi: 10.1107/S2414314624012409. eCollection 2025 Jan. IUCrdata. 2025. PMID: 39927307 Free PMC article.
-
X-ray fluorescence microscopy and X-ray absorption spectroscopy reveal the stability of the plecstatin-1 scaffold in biological model systems: comparison of Ru, Os and Ir analogues.Chem Sci. 2025 May 29;16(25):11347-11358. doi: 10.1039/d5sc02925b. eCollection 2025 Jun 25. Chem Sci. 2025. PMID: 40453796 Free PMC article.
-
Advanced Nanomaterials Functionalized with Metal Complexes for Cancer Therapy: From Drug Loading to Targeted Cellular Response.Pharmaceuticals (Basel). 2025 Jul 3;18(7):999. doi: 10.3390/ph18070999. Pharmaceuticals (Basel). 2025. PMID: 40732288 Free PMC article. Review.
References
-
- Monro S.; Colón K. L.; Yin H.; Roque J.; Konda P.; Gujar S.; Thummel R. P.; Lilge L.; Cameron C. G.; McFarland S. A. Transition Metal Complexes and Photodynamic Therapy from a Tumor-Centered Approach: Challenges, Opportunities, and Highlights from the Development of TLD1433. Chem. Rev. 2019, 119 (2), 797–828. 10.1021/acs.chemrev.8b00211. - DOI - PMC - PubMed
-
- Peyrone M. Ueber Die Einwirkung Des Ammoniaks Auf Platinchloriii. Justus Liebigs Ann. Chem. 1844, 51, 1–29. 10.1002/jlac.18440510102. - DOI
Publication types
LinkOut - more resources
Full Text Sources
