Median effective dose of intranasal dexmedetomidine for satisfactory mask induction in children undergoing examination under anaesthesia for retinoblastoma - A prospective up and down sequential allocation study
- PMID: 38435664
- PMCID: PMC10903779
- DOI: 10.4103/ija.ija_496_23
Median effective dose of intranasal dexmedetomidine for satisfactory mask induction in children undergoing examination under anaesthesia for retinoblastoma - A prospective up and down sequential allocation study
Abstract
Background and aims: Inhalational technique is used to induce anaesthesia in children without intravenous access. We aimed to determine the median effective dose (ED50) of intranasal dexmedetomidine to ensure satisfactory mask acceptance during inhalation induction in children with retinoblastoma undergoing examination under anaesthesia.
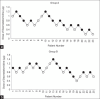
Methods: A prospective sequential allocation study was conducted in children aged 1-60 months divided into Group A (1-18 months) and Group B (18-60 months). Children were administered dexmedetomidine intranasally as premedication. Sedation was assessed using the modified Observer Assessment of Alertness and Sedation Scale until induction. Successful mask acceptance was defined as a cooperative or asleep child during inhalational induction. The starting dose of dexmedetomidine was 1 µg/kg. The next dose varied by 0.2 µg/kg depending on the outcome of this case. According to the Dixon up-and-down method, the mean of midpoints of the failure-success sequence was calculated to obtain the ED50 values.
Results: The ED50 of intranasal dexmedetomidine for satisfactory mask acceptance was 0.7 µg/kg (95% confidence interval [CI]: 0.54-0.86) in Group A (n = 23) and 0.96 µg/kg (95% CI: 0.83-1.08) in Group B (n = 25) (P = 0.020). The mean (standard deviation) duration of anaesthesia was 33.5 (14.9) minutes in group A versus 23.5 (8.48) minutes in Group B (P = 0.007).
Conclusion: ED50 was lower in children younger than 18 months than in older children. There was no difference in the time to discharge from the post-anaesthesia care unit despite the procedure being longer in smaller children.
Keywords: Children; dexmedetomidine; facemask; inhalation induction; intranasal; mask acceptance; median effective dose; sedation.
Copyright: © 2024 Indian Journal of Anaesthesia.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- Rao R, Honavar SG. Retinoblastoma. Indian J Pediatr. 2017;84:937–44. - PubMed
-
- Fabian ID, Shah V, Kapelushnik N, Naeem Z, Onadim Z, Price EA, et al. Number, frequency and time interval of examinations under anaesthesia in bilateral retinoblastoma. Graefes Arch Clin Exp Ophthalmol. 2020;258:879–86. - PubMed
-
- Yu Q, Liu Y, Sun M, Zhang J, Zhao Y, Liu F, et al. Median effective dose of intranasal dexmedetomidine sedation for transthoracic echocardiography in pediatric patients with noncyanotic congenital heart disease: An up-and-down sequential allocation trial. Pediatr Anesth. 2017;27:1108–14. - PubMed
-
- Gokhan O, Mir Hyder A. Use of intranasal dexmedetomidine as a solo sedative for magnetic resonance imaging of infants. Hosp Pediatr. 2018;141:68–71.
LinkOut - more resources
Full Text Sources