Characterization of the dispirotripiperazine derivative PDSTP as antibiotic adjuvant and antivirulence compound against Pseudomonas aeruginosa
- PMID: 38435690
- PMCID: PMC10904629
- DOI: 10.3389/fmicb.2024.1357708
Characterization of the dispirotripiperazine derivative PDSTP as antibiotic adjuvant and antivirulence compound against Pseudomonas aeruginosa
Abstract
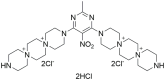
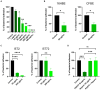
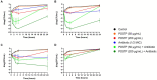
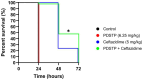
Pseudomonas aeruginosa is a major human pathogen, able to establish difficult-to-treat infections in immunocompromised and people with cystic fibrosis (CF). The high rate of antibiotic treatment failure is due to its notorious drug resistance, often mediated by the formation of persistent biofilms. Alternative strategies, capable of overcoming P. aeruginosa resistance, include antivirulence compounds which impair bacterial pathogenesis without exerting a strong selective pressure, and the use of antimicrobial adjuvants that can resensitize drug-resistant bacteria to specific antibiotics. In this work, the dispirotripiperazine derivative PDSTP, already studied as antiviral, was characterized for its activity against P. aeruginosa adhesion to epithelial cells, its antibiotic adjuvant ability and its biofilm inhibitory potential. PDSTP was effective in impairing the adhesion of P. aeruginosa to various immortalized cell lines. Moreover, the combination of clinically relevant antibiotics with the compound led to a remarkable enhancement of the antibiotic efficacy towards multidrug-resistant CF clinical strains. PDSTP-ceftazidime combination maintained its efficacy in vivo in a Galleria mellonella infection model. Finally, the compound showed a promising biofilm inhibitory activity at low concentrations when tested both in vitro and using an ex vivo pig lung model. Altogether, these results validate PDSTP as a promising compound, combining the ability to decrease P. aeruginosa virulence by impairing its adhesion and biofilm formation, with the capability to increase antibiotic efficacy against antibiotic resistant strains.
Keywords: Pseudomonas aeruginosa; antiadhesion; antibiofilm; antibiotic adjuvant; antivirulence; combination therapy; drug resistance.
Copyright © 2024 Bonacorsi, Trespidi, Scoffone, Irudal, Barbieri, Riabova, Monakhova, Makarov and Buroni.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


Similar articles
-
Antipathogenic Compounds That Are Effective at Very Low Concentrations and Have Both Antibiofilm and Antivirulence Effects against Pseudomonas aeruginosa.Microbiol Spectr. 2021 Oct 31;9(2):e0024921. doi: 10.1128/Spectrum.00249-21. Epub 2021 Sep 8. Microbiol Spectr. 2021. PMID: 34494853 Free PMC article.
-
Repurposing High-Throughput Screening Identifies Unconventional Drugs with Antibacterial and Antibiofilm Activities against Pseudomonas aeruginosa under Experimental Conditions Relevant to Cystic Fibrosis.Microbiol Spectr. 2023 Aug 17;11(4):e0035223. doi: 10.1128/spectrum.00352-23. Epub 2023 Jun 12. Microbiol Spectr. 2023. PMID: 37306577 Free PMC article.
-
Antibiotic-Potentiating Effect of Some Bioactive Natural Products against Planktonic Cells, Biofilms, and Virulence Factors of Pseudomonas aeruginosa.Biomed Res Int. 2023 Aug 23;2023:9410609. doi: 10.1155/2023/9410609. eCollection 2023. Biomed Res Int. 2023. PMID: 37663785 Free PMC article.
-
Pseudomonas aeruginosa chromosomal beta-lactamase in patients with cystic fibrosis and chronic lung infection. Mechanism of antibiotic resistance and target of the humoral immune response.APMIS Suppl. 2003;(116):1-47. APMIS Suppl. 2003. PMID: 14692154 Review.
-
Standard versus biofilm antimicrobial susceptibility testing to guide antibiotic therapy in cystic fibrosis.Cochrane Database Syst Rev. 2020 Jun 10;6(6):CD009528. doi: 10.1002/14651858.CD009528.pub5. Cochrane Database Syst Rev. 2020. PMID: 32520436 Free PMC article.
Cited by
-
Efficacy of dispirotripiperazine PDSTP in a golden Syrian hamster model of SARS-CoV-2 infection.Front Microbiol. 2025 Mar 10;16:1546946. doi: 10.3389/fmicb.2025.1546946. eCollection 2025. Front Microbiol. 2025. PMID: 40130242 Free PMC article.
References
-
- Alcalá-Franco B., Montanari S., Cigana C., Bertoni G., Oliver A., Bragonzi A. (2012). Antibiotic pressure compensates the biological cost associated with Pseudomonas aeruginosa hypermutable phenotypes in vitro and in a murine model of chronic airways infection. J. Antimicrob. Chemother. 67, 962–969. doi: 10.1093/jac/dkr587 - DOI - PubMed
-
- Benthall G., Touzel R. E., Hind C. K., Titball R. W., Sutton J. M., Thomas R. J., et al. . (2015). Evaluation of antibiotic efficacy against infections caused by planktonic or biofilm cultures of Pseudomonas aeruginosa and Klebsiella pneumoniae in galleria mellonella. Int. J. Antimicrob. Agents 46, 538–545. doi: 10.1016/j.ijantimicag.2015.07.014 - DOI - PubMed
LinkOut - more resources
Full Text Sources

