Trafficking of hormones and trophic factors to secretory and extracellular vesicles: a historical perspective and new hypothesis
- PMID: 38435713
- PMCID: PMC10906782
- DOI: 10.20517/evcna.2023.34
Trafficking of hormones and trophic factors to secretory and extracellular vesicles: a historical perspective and new hypothesis
Abstract
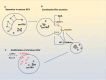
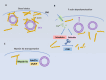
It is well known that peptide hormones and neurotrophic factors are intercellular messengers that are packaged into secretory vesicles in endocrine cells and neurons and released by exocytosis upon the stimulation of the cells in a calcium-dependent manner. These secreted molecules bind to membrane receptors, which then activate signal transduction pathways to mediate various endocrine/trophic functions. Recently, there is evidence that these molecules are also in extracellular vesicles, including small extracellular vesicles (sEVs), which appear to be taken up by recipient cells. This finding raised the hypothesis that they may have functions differentiated from their classical secretory hormone/neurotrophic factor actions. In this article, the historical perspective and updated mechanisms for the sorting and packaging of hormones and neurotrophic factors into secretory vesicles and their transport in these organelles for release at the plasma membrane are reviewed. In contrast, little is known about the packaging of hormones and neurotrophic factors into extracellular vesicles. One proposal is that these molecules could be sorted at the trans-Golgi network, which then buds to form Golgi-derived vesicles that can fuse to endosomes and subsequently form intraluminal vesicles. They are then taken up by multivesicular bodies to form extracellular vesicles, which are subsequently released. Other possible mechanisms for packaging RSP proteins into sEVs are discussed. We highlight some studies in the literature that suggest the dual vesicular pathways for the release of hormones and neurotrophic factors from the cell may have some physiological significance in intercellular communication.
Keywords: Hormone trafficking; endocrine cells; exosomes; extracellular vesicles; neurons; sEV; trophic factor.
Conflict of interest statement
Conflicts of interest The authors declare that they have no conflicts of interest.
Figures





Similar articles
-
TRP Channel Trafficking.In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 23. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 23. PMID: 21204515 Free Books & Documents. Review.
-
Gliocrine System: Astroglia as Secretory Cells of the CNS.Adv Exp Med Biol. 2019;1175:93-115. doi: 10.1007/978-981-13-9913-8_4. Adv Exp Med Biol. 2019. PMID: 31583585 Free PMC article. Review.
-
Exosomes: a bubble ride for prions?Traffic. 2005 Jan;6(1):10-7. doi: 10.1111/j.1600-0854.2004.00247.x. Traffic. 2005. PMID: 15569241 Review.
-
CD63 Regulates Epstein-Barr Virus LMP1 Exosomal Packaging, Enhancement of Vesicle Production, and Noncanonical NF-κB Signaling.J Virol. 2017 Feb 14;91(5):e02251-16. doi: 10.1128/JVI.02251-16. Print 2017 Mar 1. J Virol. 2017. PMID: 27974566 Free PMC article.
-
Adaptor Protein CD2AP and L-type Lectin LMAN2 Regulate Exosome Cargo Protein Trafficking through the Golgi Complex.J Biol Chem. 2016 Dec 2;291(49):25462-25475. doi: 10.1074/jbc.M116.729202. Epub 2016 Oct 20. J Biol Chem. 2016. PMID: 27765817 Free PMC article.
Cited by
-
Extracellular vesicles and macrophages in tumor microenvironment: Impact on cervical cancer.Heliyon. 2024 Jul 26;10(15):e35063. doi: 10.1016/j.heliyon.2024.e35063. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39165926 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
