Self-assembly of PEG-PPS polymers and LL-37 peptide nanomicelles improves the oxidative microenvironment and promotes angiogenesis to facilitate chronic wound healing
- PMID: 38435813
- PMCID: PMC10905545
- DOI: 10.1002/btm2.10619
Self-assembly of PEG-PPS polymers and LL-37 peptide nanomicelles improves the oxidative microenvironment and promotes angiogenesis to facilitate chronic wound healing
Erratum in
-
Correction to "Self-assembly of PEG-PPS polymers and LL-37 peptide nanomicelles improves the oxidative microenvironment and promotes angiogenesis to facilitate chronic wound healing".Bioeng Transl Med. 2024 Sep 9;9(6):e10718. doi: 10.1002/btm2.10718. eCollection 2024 Nov. Bioeng Transl Med. 2024. PMID: 39545080 Free PMC article.
Abstract
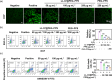
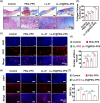
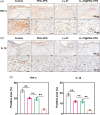
Refractory diabetic wounds are associated with high incidence, mortality, and recurrence rates and are a devastating and rapidly growing clinical problem. However, treating these wounds is difficult owing to uncontrolled inflammatory microenvironments and defective angiogenesis in the affected areas, with no established effective treatment to the best of our knowledge. Herein, we optimized a dual functional therapeutic agent based on the assembly of LL-37 peptides and diblock copolymer poly(ethylene glycol)-poly(propylene sulfide) (PEG-PPS). The incorporation of PEG-PPS enabled responsive or controlled LL-37 peptide release in the presence of reactive oxygen species (ROS). LL-37@PEG-PPS nanomicelles not only scavenged excessive ROS to improve the microenvironment for angiogenesis but also released LL-37 peptides and protected them from degradation, thereby robustly increasing angiogenesis. Diabetic wounds treated with LL-37@PEG-PPS exhibited accelerated and high-quality wound healing in vivo. This study shows that LL-37@PEG-PPS can restore beneficial angiogenesis in the wound microenvironment by continuously providing angiogenesis-promoting signals. Thus, it may be a promising drug for improving chronic refractory wound healing.
Keywords: LL‐37@PEG–PPS nanomicelles; angiogenesis; diabetic wound healing; inflammatory microenvironment.
© 2023 The Authors. Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Promoting Re-epithelialization in an oxidative diabetic wound microenvironment using self-assembly of a ROS-responsive polymer and P311 peptide micelles.Acta Biomater. 2022 Oct 15;152:425-439. doi: 10.1016/j.actbio.2022.09.017. Epub 2022 Sep 13. Acta Biomater. 2022. PMID: 36113723
-
Correction to "Self-assembly of PEG-PPS polymers and LL-37 peptide nanomicelles improves the oxidative microenvironment and promotes angiogenesis to facilitate chronic wound healing".Bioeng Transl Med. 2024 Sep 9;9(6):e10718. doi: 10.1002/btm2.10718. eCollection 2024 Nov. Bioeng Transl Med. 2024. PMID: 39545080 Free PMC article.
-
Combined ROS Sensitive PEG-PPS-PEG with Peptide Agonist for Effective Target Therapy in Mouse Model.Int J Nanomedicine. 2024 Sep 5;19:9109-9120. doi: 10.2147/IJN.S471036. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39253061 Free PMC article.
-
PF-PEG@ASIV-EXO Hydrogel Accelerates Diabetic Wound Healing by Ferroptosis Resistance and Promoting Angiogenesis.ACS Biomater Sci Eng. 2024 Oct 14;10(10):6263-6285. doi: 10.1021/acsbiomaterials.4c00692. Epub 2024 Sep 23. ACS Biomater Sci Eng. 2024. PMID: 39311841
-
A nanofibrous membrane loaded with doxycycline and printed with conductive hydrogel strips promotes diabetic wound healing in vivo.Acta Biomater. 2022 Oct 15;152:60-73. doi: 10.1016/j.actbio.2022.08.048. Epub 2022 Aug 30. Acta Biomater. 2022. PMID: 36049625
Cited by
-
Pressure injuries and biofilms: Microbiome, model systems and therapies.Wound Repair Regen. 2025 Jan-Feb;33(1):e70005. doi: 10.1111/wrr.70005. Wound Repair Regen. 2025. PMID: 39949184 Free PMC article. Review.
-
Bioengineering strategies targeting angiogenesis: Innovative solutions for osteonecrosis of the femoral head.J Tissue Eng. 2025 Jan 24;16:20417314241310541. doi: 10.1177/20417314241310541. eCollection 2025 Jan-Dec. J Tissue Eng. 2025. PMID: 39866964 Free PMC article. Review.
-
A multifunctional dihydromyricetin-loaded hydrogel for the sequential modulation of diabetic wound healing and glycemic control.Burns Trauma. 2025 Mar 19;13:tkaf024. doi: 10.1093/burnst/tkaf024. eCollection 2025. Burns Trauma. 2025. PMID: 40757164 Free PMC article.
References
-
- Hassanshahi A, Hassanshahi M, Khabbazi S, et al. Adipose‐derived stem cells for wound healing. J Cell Physiol. 2019;234:7903‐7914. - PubMed
-
- Jones RE, Foster DS, Longaker MT. Management of chronic wounds‐2018. JAMA. 2018;320:1481‐1482. - PubMed
-
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736‐1743. - PubMed
-
- Cho H, Blatchley MR, Duh EJ, Gerecht S. Acellular and cellular approaches to improve diabetic wound healing. Adv Drug Deliv Rev. 2019;146:267‐288. - PubMed
LinkOut - more resources
Full Text Sources

