Functional characterization of GhNAC2 promoter conferring hormone- and stress-induced expression: a potential tool to improve growth and stress tolerance in cotton
- PMID: 38435854
- PMCID: PMC10901759
- DOI: 10.1007/s12298-024-01411-2
Functional characterization of GhNAC2 promoter conferring hormone- and stress-induced expression: a potential tool to improve growth and stress tolerance in cotton
Abstract
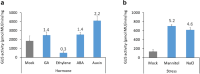
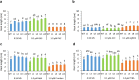
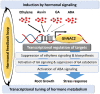
The GhNAC2 transcription factor identified from G. herbaceum improves root growth and drought tolerance through transcriptional reprogramming of phytohormone signaling. The promoter of such a versatile gene could serve as an important genetic engineering tool for biotechnological application. In this study, we identified and characterized the promoter of GhNAC2 to understand its regulatory mechanism. GhNAC2 transcription factor increased in root tissues in response to GA, ethylene, auxin, ABA, mannitol, and NaCl. In silico analysis revealed an overrepresentation of cis-regulatory elements associated with hormone signaling, stress responses and root-, pollen-, and seed-specific promoter activity. To validate their role in GhNAC2 function/regulation, an 870-bp upstream regulatory sequence was fused with the GUS reporter gene (uidA) and expressed in Arabidopsis and cotton hairy roots for in planta characterization. Histochemical GUS staining indicated localized expression in root tips, root elongation zone, root primordia, and reproductive tissues under optimal growth conditions. Mannitol, NaCl, auxin, GA, and ABA, induced the promoter-driven GUS expression in all tissues while ethylene suppressed the promoter activity. The results show that the 870 nt fragment of the GhNAC2 promoter drives root-preferential expression and responds to phytohormonal and stress signals. In corroboration with promoter regulation, GA and ethylene pathways differentially regulated root growth in GhNAC2-expressing Arabidopsis. The findings suggest that differential promoter activity governs the expression of GhNAC2 in root growth and stress-related functions independently through specific promoter elements. This multifarious promoter can be utilized to develop yield and climate resilience in cotton by expanding the options to control gene regulation.
Supplementary information: The online version contains supplementary material available at 10.1007/s12298-024-01411-2.
Keywords: Cotton; Gene regulation; GhNAC2; Hormone signaling; Inducible promoter; Root.
© Prof. H.S. Srivastava Foundation for Science and Society 2024. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestAll authors declare not having competing or conflicting interests.
Figures





Similar articles
-
Expression of GhNAC2 from G. herbaceum, improves root growth and imparts tolerance to drought in transgenic cotton and Arabidopsis.Sci Rep. 2016 Apr 26;6:24978. doi: 10.1038/srep24978. Sci Rep. 2016. PMID: 27113714 Free PMC article.
-
The Functions of an NAC Transcription Factor, GhNAC2-A06, in Cotton Response to Drought Stress.Plants (Basel). 2023 Nov 2;12(21):3755. doi: 10.3390/plants12213755. Plants (Basel). 2023. PMID: 37960109 Free PMC article.
-
GmPRP2 promoter drives root-preferential expression in transgenic Arabidopsis and soybean hairy roots.BMC Plant Biol. 2014 Sep 16;14:245. doi: 10.1186/s12870-014-0245-z. BMC Plant Biol. 2014. PMID: 25224536 Free PMC article.
-
Harlequin (hlq) and short blue root (sbr), two Arabidopsis mutants that ectopically express an abscisic acid- and auxin-inducible transgenic carrot promoter and have pleiotropic effects on morphogenesis.Plant Mol Biol. 2002 May;49(1):93-105. doi: 10.1023/a:1014472417150. Plant Mol Biol. 2002. PMID: 12008902
-
A receptor-like kinase gene (GbRLK) from Gossypium barbadense enhances salinity and drought-stress tolerance in Arabidopsis.BMC Plant Biol. 2013 Aug 6;13:110. doi: 10.1186/1471-2229-13-110. BMC Plant Biol. 2013. PMID: 23915077 Free PMC article.
References
-
- Asif MH, Dhawan P, Nath P. A simple procedure for the isolation of high quality RNA from ripening banana fruit. Plant Mol Biol Rep. 2000;18(2):109–115. doi: 10.1007/BF02824018. - DOI
-
- Biłas R, Szafran K, Hnatuszko-Konka K, Kononowicz AK. Cis-regulatory elements used to control gene expression in plants. Plant Cell Tissue Organ Cult. 2016;127(2):269–287. doi: 10.1007/s11240-016-1057-7. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
