Delayed Irreversible Fanconi Syndrome Associated With Vertebral Fracture After Tenofovir Discontinuation
- PMID: 38435900
- PMCID: PMC10905307
- DOI: 10.7759/cureus.53280
Delayed Irreversible Fanconi Syndrome Associated With Vertebral Fracture After Tenofovir Discontinuation
Abstract
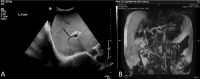
The use of tenofovir disoproxil fumarate (TDF) as an antiretroviral agent has been reported to adversely affect both renal tubules and bone health, leading to pathological fractures. While such an effect is largely reversible, substituting TDF with tenofovir alafenamide (TAF) might result in lower rates of adverse events with the preservation of tenofovir effectiveness. We report a case of a 40-year-old lady with HIV infection who had a vertebral fragility fracture secondary to TDF-associated Fanconi syndrome. The syndrome developed four years after TDF cessation and switching to TAF. Other etiologies for decreased bone mass were excluded, and the diagnosis of Fanconi syndrome was established based on her bone mineral density (BMD) and urine parameters. She was treated conservatively with active vitamin D, calcium, and progesterone/estrogen combination, but her phosphate wasting persisted despite switching to TAF; this likely represents a delayed irreversible effect of TDF on the patient's bone remodeling. This case report highlights the chronic sequelae of TDF therapy and the importance of monitoring for and early detection of renal tubulopathy and osteoporotic fractures in this patient population.
Keywords: bone mineral density (bmd); human immunodeficiency virus (hiv); osteoporotic fractures; tenofovir alafenamide (taf); tenofovir disoproxil fumarate (tdf).
Copyright © 2024, Qorban et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Tenofovir Alafenamide Versus Tenofovir Disoproxil Fumarate in the First Protease Inhibitor-Based Single-Tablet Regimen for Initial HIV-1 Therapy: A Randomized Phase 2 Study.J Acquir Immune Defic Syndr. 2015 Aug 1;69(4):439-45. doi: 10.1097/QAI.0000000000000618. J Acquir Immune Defic Syndr. 2015. PMID: 25867913 Clinical Trial.
-
Recovery of Tenofovir-induced Nephrotoxicity following Switch from Tenofovir Disoproxil Fumarate to Tenofovir Alafenamide in Human Immunodeficiency Virus-Positive Patients.Infect Chemother. 2020 Sep;52(3):381-388. doi: 10.3947/ic.2020.52.3.381. Epub 2020 Jul 16. Infect Chemother. 2020. PMID: 32757496 Free PMC article.
-
Tenofovir alafenamide versus tenofovir disoproxil fumarate: is there a true difference in efficacy and safety?J Virus Erad. 2018 Apr 1;4(2):72-79. doi: 10.1016/S2055-6640(20)30248-X. J Virus Erad. 2018. PMID: 29682298 Free PMC article.
-
Efficacy and safety of the regimens containing tenofovir alafenamide versus tenofovir disoproxil fumarate in fixed-dose single-tablet regimens for initial treatment of HIV-1 infection: A meta-analysis of randomized controlled trials.Int J Infect Dis. 2020 Apr;93:108-117. doi: 10.1016/j.ijid.2020.01.035. Epub 2020 Jan 25. Int J Infect Dis. 2020. PMID: 31988012 Review.
-
Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF).Biochem Pharmacol. 2016 Nov 1;119:1-7. doi: 10.1016/j.bcp.2016.04.015. Epub 2016 Apr 29. Biochem Pharmacol. 2016. PMID: 27133890 Review.
References
-
- HAART in HIV/AIDS treatments: future trends. Lu DY, Wu HY, Yarla NS, Xu B, Ding J, Lu TR. Infect Disord Drug Targets. 2018;18:15–22. - PubMed
-
- Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. [ Nov; 2023 ]. 2022. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult... https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult...
-
- Efficacy and safety of the regimens containing tenofovir alafenamide versus tenofovir disoproxil fumarate in fixed-dose single-tablet regimens for initial treatment of HIV-1 infection: a meta-analysis of randomized controlled trials. Tao X, Lu Y, Zhou Y, Zhang L, Chen Y. Int J Infect Dis. 2020;93:108–117. - PubMed
-
- Virologically suppressed HIV-infected patients on TDF-containing regimens significantly benefit from switching to TAF-containing regimens: a meta-analysis of randomized controlled trials. Tao X, Lu Y, Zhou Y, Huang Y, Chen Y. Int J Infect Dis. 2019;87:43–53. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
