Physiological role of potassium channels in mammalian germ cell differentiation, maturation, and capacitation
- PMID: 38436215
- PMCID: PMC11815548
- DOI: 10.1111/andr.13606
Physiological role of potassium channels in mammalian germ cell differentiation, maturation, and capacitation
Abstract
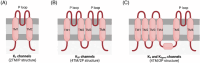
Background: Ion channels are essential for differentiation and maturation of germ cells, and even for fertilization in mammals. Different types of potassium channels have been identified, which are grouped into voltage-gated channels (Kv), ligand-gated channels (Kligand), inwardly rectifying channels (Kir), and tandem pore domain channels (K2P).
Material-methods: The present review includes recent findings on the role of potassium channels in sperm physiology of mammals.
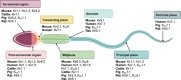
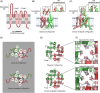
Results-discussion: While most studies conducted thus far have been focused on the physiological role of voltage- (Kv1, Kv3, and Kv7) and calcium-gated channels (SLO1 and SLO3) during sperm capacitation, especially in humans and rodents, little data about the types of potassium channels present in the plasma membrane of differentiating germ cells exist. In spite of this, recent evidence suggests that the content and regulation mechanisms of these channels vary throughout spermatogenesis. Potassium channels are also essential for the regulation of sperm cell volume during epididymal maturation and for preventing premature membrane hyperpolarization. It is important to highlight that the nature, biochemical properties, localization, and regulation mechanisms of potassium channels are species-specific. In effect, while SLO3 is the main potassium channel involved in the K+ current during sperm capacitation in rodents, different potassium channels are implicated in the K+ outflow and, thus, plasma membrane hyperpolarization during sperm capacitation in other mammalian species, such as humans and pigs.
Conclusions: Potassium conductance is essential for male fertility, not only during sperm capacitation but throughout the spermiogenesis and epididymal maturation.
Keywords: cell volume; epididymal maturation; plasma membrane potential; sperm capacitation; spermatogenesis.
© 2024 The Authors. Andrology published by Wiley Periodicals LLC on behalf of American Society of Andrology and European Academy of Andrology.
Conflict of interest statement
The authors declare that they have no conflicts of interest regarding this review article.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

