Re-framing the importance of Group B Streptococcus as a gut-resident pathobiont
- PMID: 38436256
- PMCID: PMC11392526
- DOI: 10.1128/iai.00478-23
Re-framing the importance of Group B Streptococcus as a gut-resident pathobiont
Abstract
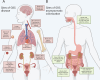
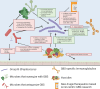
Streptococcus agalactiae (Group B Streptococcus, GBS) is a Gram-positive bacterial species that causes disease in humans across the lifespan. While antibiotics are used to mitigate GBS infections, it is evident that antibiotics disrupt human microbiomes (which can predispose people to other diseases later in life), and antibiotic resistance in GBS is on the rise. Taken together, these unintended negative impacts of antibiotics highlight the need for precision approaches for minimizing GBS disease. One possible approach involves selectively depleting GBS in its commensal niches before it can cause disease at other body sites or be transmitted to at-risk individuals. One understudied commensal niche of GBS is the adult gastrointestinal (GI) tract, which may predispose colonization at other body sites in individuals at risk for GBS disease. However, a better understanding of the host-, microbiome-, and GBS-determined variables that dictate GBS GI carriage is needed before precise GI decolonization approaches can be developed. In this review, we synthesize current knowledge of the diverse body sites occupied by GBS as a pathogen and as a commensal. We summarize key molecular factors GBS utilizes to colonize different host-associated niches to inform future efforts to study GBS in the GI tract. We also discuss other GI commensals that are pathogenic in other body sites to emphasize the broader utility of precise de-colonization approaches for mitigating infections by GBS and other bacterial pathogens. Finally, we highlight how GBS treatments could be improved with a more holistic understanding of GBS enabled by continued GI-focused study.
Keywords: Streptococcus agalactiae; gut microbiome; human microbiome; microbial ecology; pathogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Francois Watkins LK, McGee L, Schrag SJ, Beall B, Jain JH, Pondo T, Farley MM, Harrison LH, Zansky SM, Baumbach J, Lynfield R, Snippes Vagnone P, Miller LA, Schaffner W, Thomas AR, Watt JP, Petit S, Langley GE. 2019. Epidemiology of invasive Group B Streptococcal infections among nonpregnant adults in the United States, 2008-2016. JAMA Intern Med 179:479–488. doi:10.1001/jamainternmed.2018.7269 - DOI - PMC - PubMed
-
- Deutscher M, Lewis M, Zell ER, Taylor TH, Van Beneden C, Schrag S, Active Bacterial Core Surveillance Team . 2011. Incidence and severity of invasive Streptococcus pneumoniae, Group A Streptococcus, and Group B Streptococcus infections among pregnant and postpartum women. Clin Infect Dis 53:114–123. doi:10.1093/cid/cir325 - DOI - PubMed
-
- Gonçalves BP, Procter SR, Paul P, Chandna J, Lewin A, Seedat F, Koukounari A, Dangor Z, Leahy S, Santhanam S, et al. . 2022. Group B Streptococcus infection during pregnancy and infancy: estimates of regional and global burden. Lancet Glob Health 10:e807–e819. doi:10.1016/S2214-109X(22)00093-6 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

