One-month DAPT with ticagrelor and aspirin for patients undergoing coronary artery bypass grafting: rationale and design of the randomised, multicentre, double-blind, placebo-controlled ODIN trial
- PMID: 38436365
- PMCID: PMC10905196
- DOI: 10.4244/EIJ-D-23-00699
One-month DAPT with ticagrelor and aspirin for patients undergoing coronary artery bypass grafting: rationale and design of the randomised, multicentre, double-blind, placebo-controlled ODIN trial
Abstract
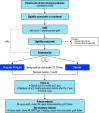
The optimal antiplatelet strategy after coronary artery bypass graft (CABG) surgery in patients with chronic coronary syndromes (CCS) is unclear. Adding the P2Y12 inhibitor, ticagrelor, to low-dose aspirin for 1 year is associated with a reduction in graft failure, particularly saphenous vein grafts, at the expense of an increased risk of clinically important bleeding. As the risk of thrombotic graft failure and ischaemic events is highest early after CABG surgery, a better risk-to-benefit profile may be attained with short-term dual antiplatelet therapy followed by single antiplatelet therapy. The One Month Dual Antiplatelet Therapy With Ticagrelor in Coronary Artery Bypass Graft Patients (ODIN) trial is a prospective, randomised, double-blind, placebo-controlled, international, multicentre study of 700 subjects that will evaluate the effect of short-term dual antiplatelet therapy with ticagrelor plus low-dose aspirin after CABG in patients with CCS. Patients will be randomised 1:1 to ticagrelor 90 mg twice daily or matching placebo, in addition to aspirin 75-150 mg once daily for 1 month; after the first month, antiplatelet therapy will be continued with aspirin alone. The primary endpoint is a hierarchical composite of all-cause death, stroke, myocardial infarction, revascularisation and graft failure at 1 year. The key secondary endpoint is a hierarchical composite of all-cause death, stroke, myocardial infarction, Bleeding Academic Research Consortium (BARC) type 3 bleeding, revascularisation and graft failure at 1 year (net clinical benefit). ODIN will report whether the addition of ticagrelor to low-dose aspirin for 1 month after CABG reduces ischaemic events and provides a net clinical benefit in patients with CCS. (ClinicalTrials.gov: NCT05997693).
Conflict of interest statement
D.J. Angiolillo has received consulting fees or honoraria from Abbott, Amgen, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Daiichi Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, Novartis, PhaseBio, PLx Pharma, Pfizer, Sanofi, and Vectura; his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova (now Alta Biomaterials), CSL Behring, Daiichi Sankyo, Eisai, Eli Lilly, Gilead Sciences, Idorsia, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, and the Scott R. MacKenzie Foundation. D.L. Bhatt has had advisory board roles with ANGIOWave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier, High Enroll, Janssen, Level Ex, McKinsey & Company, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, and Stasys; he has held board of directors’ positions with ANGIOWave (stock options), Boston VA Research Institute, Bristol-Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock), Society of Cardiovascular Patient Care, and TobeSoft; he has held Chair roles including inaugural Chair for American Heart Association Quality Oversight Committee; he has held consultant roles with Broadview Ventures, and Hims; and has been on data monitoring committees, including for Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research, Boston Scientific, Cleveland Clinic, Contego Medical, Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, Novartis, Population Health Research Institute, and Rutgers University; and has received honoraria from various organisations; he has also held other roles with Clinical Cardiology, NCDR-ACTION Registry Steering Committee, and VA CART Research and Publications Committee; he has held a patent for Sotagliflozin; he has received research funding from numerous entities, including AstraZeneca; he has received royalties from Elsevier; he has been a site co-investigator for multiple companies; he has been a trustee for the American College of Cardiology; and he has carried out unfunded research with FlowCo and Takeda. S.E. Fremes has received grant support from CIHR, NIH, Medtronic, Boston Scientific, and Amgen. S.V. Rao has received research funding from NHLBI. J.A. Spertus has provided consultancy services to Alnylam, AstraZeneca, Bayer, Merck, Janssen, Bristol-Myers Squibb, Edwards Lifesciences, Kineksia, 4D Medical, Terumo, Cytokinetics, Imbria, and UnitedHealthcare; he has received research grants from Bristol-Myers Squibb, Abbott, and Janssen; he has held ownership of copyrights to the Seattle Angina Questionnaire, Kansas City Cardiomyopathy Questionnaire, and Peripheral Artery Questionnaire; and he has been on the board of directors for Blue Cross Blue Shield of Kansas City. The other authors have no conflicts of interest to declare.
Figures
References
-
- Alkhouli M, Alqahtani F, Kalra A, Gafoor S, Alhajji M, Alreshidan M, Holmes DR, Lerman A. Trends in Characteristics and Outcomes of Patients Undergoing Coronary Revascularization in the United States, 2003-2016. JAMA Netw Open. 2020;3:e1921326. - PubMed
-
- Schwann TA, Habib RH, Wallace A, Shahian DM, O’Brien S, Jacobs JP, Puskas JD, Kurlansky PA, Engoren MC, Tranbaugh RF, Bonnell MR. Operative Outcomes of Multiple-Arterial Versus Single-Arterial Coronary Bypass Grafting. Ann Thorac Surg. 2018;105:1109–19. - PubMed
-
- Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferović PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO. 2018 ESC/EACTS Guidelines on myocardial revascularization. EuroIntervention. 2019;14:1435–534. - PubMed
-
- Sandner S, Redfors B, Angiolillo DJ, Audisio K, Fremes SE, Janssen PWA, Kulik A, Mehran R, Peper J, Ruel M, Saw J, Soletti GJ, Starovoytov A, Ten Berg, Willemsen LM, Zhao Q, Zhu Y, Gaudino M. Association of Dual Antiplatelet Therapy With Ticagrelor With Vein Graft Failure After Coronary Artery Bypass Graft Surgery: A Systematic Review and Meta-analysis. JAMA. 2022;328:554–62. - PMC - PubMed
-
- Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–47. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials