Obesogenic diet induces circuit-specific memory deficits in mice
- PMID: 38436653
- PMCID: PMC10911750
- DOI: 10.7554/eLife.80388
Obesogenic diet induces circuit-specific memory deficits in mice
Abstract
Obesity is associated with neurocognitive dysfunction, including memory deficits. This is particularly worrisome when obesity occurs during adolescence, a maturational period for brain structures critical for cognition. In rodent models, we recently reported that memory impairments induced by obesogenic high-fat diet (HFD) intake during the periadolescent period can be reversed by chemogenetic manipulation of the ventral hippocampus (vHPC). Here, we used an intersectional viral approach in HFD-fed male mice to chemogenetically inactivate specific vHPC efferent pathways to nucleus accumbens (NAc) or medial prefrontal cortex (mPFC) during memory tasks. We first demonstrated that HFD enhanced activation of both pathways after training and that our chemogenetic approach was effective in normalizing this activation. Inactivation of the vHPC-NAc pathway rescued HFD-induced deficits in recognition but not location memory. Conversely, inactivation of the vHPC-mPFC pathway restored location but not recognition memory impairments produced by HFD. Either pathway manipulation did not affect exploration or anxiety-like behaviour. These findings suggest that HFD intake throughout adolescence impairs different types of memory through overactivation of specific hippocampal efferent pathways and that targeting these overactive pathways has therapeutic potential.
Keywords: adolescence; connectivity; high-fat diet; hippocampus; mouse; neuroscience; obesity; pathways.
© 2024, Bakoyiannis, Ducourneau et al.
Conflict of interest statement
IB, ED, MN, AF, CD, CB, EC, PT, GF No competing interests declared
Figures
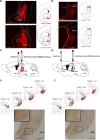

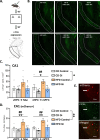



Update of
- doi: 10.1101/2022.06.20.496841
References
-
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. - DOI - PMC - PubMed
-
- André C, Dinel AL, Ferreira G, Layé S, Castanon N. Diet-induced obesity progressively alters cognition, anxiety-like behavior and lipopolysaccharide-induced depressive-like behavior: focus on brain indoleamine 2,3-dioxygenase activation. Brain, Behavior, and Immunity. 2014;41:10–21. doi: 10.1016/j.bbi.2014.03.012. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

