Effectiveness and tolerability of brivaracetam in patients with epilepsy stratified by comorbidities and etiology in the real world: 12-month subgroup data from the international EXPERIENCE pooled analysis
- PMID: 38436680
- PMCID: PMC11136785
- DOI: 10.1007/s00415-024-12253-z
Effectiveness and tolerability of brivaracetam in patients with epilepsy stratified by comorbidities and etiology in the real world: 12-month subgroup data from the international EXPERIENCE pooled analysis
Abstract
Objective: To assess the effectiveness and tolerability of brivaracetam (BRV) in adults with epilepsy by specific comorbidities and epilepsy etiologies.
Methods: EXPERIENCE/EPD332 was a pooled analysis of individual patient records from several non-interventional studies of patients with epilepsy initiating BRV in clinical practice. Outcomes included ≥ 50% reduction from baseline in seizure frequency, seizure freedom (no seizures within prior 3 months), continuous seizure freedom (no seizures since baseline), BRV discontinuation, and treatment-emergent adverse events (TEAEs) at 3, 6, and 12 months. Analyses were performed for all adult patients (≥ 16 years of age) and stratified by comorbidity and by etiology at baseline (patients with cognitive/learning disability [CLD], psychiatric comorbidity, post-stroke epilepsy, brain tumor-related epilepsy [BTRE], and traumatic brain injury-related epilepsy [TBIE]).
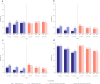
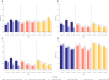
Results: At 12 months, ≥ 50% seizure reduction was achieved in 35.6% (n = 264), 38.7% (n = 310), 41.7% (n = 24), 34.1% (n = 41), and 50.0% (n = 28) of patients with CLD, psychiatric comorbidity, post-stroke epilepsy, BTRE, and TBIE, respectively; and continuous seizure freedom was achieved in 5.7% (n = 318), 13.7% (n = 424), 29.4% (n = 34), 11.4% (n = 44), and 13.8% (n = 29), respectively. During the study follow-up, in patients with CLD, psychiatric comorbidity, post-stroke epilepsy, BTRE, and TBIE, 37.1% (n = 403), 30.7% (n = 605), 33.3% (n = 51), 39.7% (n = 68), and 27.1% (n = 49) of patients discontinued BRV, respectively; and TEAEs since prior visit at 12 months were reported in 11.3% (n = 283), 10.0% (n = 410), 16.7% (n = 36), 12.5% (n = 48), and 3.0% (n = 33), respectively.
Conclusions: BRV as prescribed in the real world is effective and well tolerated among patients with CLD, psychiatric comorbidity, post-stroke epilepsy, BTRE, and TBIE.
Keywords: Brivaracetam; Comorbidity; Effectiveness; Etiology; Real world; Tolerability.
© 2024. The Author(s).
Conflict of interest statement
JPS has received research funding from Biogen, Department of Defense, Eisai, GW Pharmaceuticals companies, National Institutes of Health, National Science Foundation, NeuroPace, Serina Therapeutics, Shor Foundation for Epilepsy Research, State of Alabama General Funds, and UCB Pharma; has served as a consultant or advisory board member for Elite Medical Experts, GW Pharmaceuticals, LivaNova, Lundbeck, Medical Association of the State of Alabama, NeuroPace, Serina Therapeutics, SK Life Science, and UCB Pharma; has served as an investigator on GW Research Ltd trials; and is an editorial board member for
Figures
Similar articles
-
Brivaracetam effectiveness and tolerability in older and younger adults with epilepsy: EXPERIENCE, a pooled analysis of international data from retrospective studies.Epilepsy Behav. 2024 Sep;158:109922. doi: 10.1016/j.yebeh.2024.109922. Epub 2024 Jul 5. Epilepsy Behav. 2024. PMID: 38970892
-
Effectiveness and Tolerability of 12-Month Brivaracetam in the Real World: EXPERIENCE, an International Pooled Analysis of Individual Patient Records.CNS Drugs. 2023 Sep;37(9):819-835. doi: 10.1007/s40263-023-01033-4. Epub 2023 Sep 9. CNS Drugs. 2023. PMID: 37684497 Free PMC article. Review.
-
BRIVEST: A 'real-world' observational, single-centre study investigating the efficacy, safety and tolerability of Brivaracetam.Epilepsy Behav. 2023 Jan;138:108985. doi: 10.1016/j.yebeh.2022.108985. Epub 2022 Nov 25. Epilepsy Behav. 2023. PMID: 36442261 Clinical Trial.
-
Effectiveness and tolerability of adjunctive brivaracetam in patients with focal seizures: Second interim analysis of 6-month data from a prospective observational study in Europe.Epilepsy Res. 2020 Sep;165:106329. doi: 10.1016/j.eplepsyres.2020.106329. Epub 2020 Apr 9. Epilepsy Res. 2020. PMID: 32623096
-
Adjunctive brivaracetam for patients with refractory partial seizures: A meta-analysis of randomized placebo-controlled trials.Epilepsy Res. 2015 Aug;114:59-65. doi: 10.1016/j.eplepsyres.2015.04.017. Epub 2015 May 1. Epilepsy Res. 2015. PMID: 26088886 Review.
Cited by
-
Brivaracetam: Pharmacology, Clinical Efficacy, and Safety in Epilepsy.J Epilepsy Res. 2025 Jun 10;15(1):42-55. doi: 10.14581/jer.25005. eCollection 2025 Jun. J Epilepsy Res. 2025. PMID: 40568060 Free PMC article. Review.
-
BRIVA-ONE study: 12-month outcomes of brivaracetam monotherapy in clinical practice.Epilepsia Open. 2024 Dec;9(6):2429-2442. doi: 10.1002/epi4.13078. Epub 2024 Oct 29. Epilepsia Open. 2024. PMID: 39470722 Free PMC article.
-
Brivaracetam use in clinical practice: a Delphi consensus on its role as first add-on therapy in focal epilepsy and beyond.Neurol Sci. 2024 Sep;45(9):4519-4527. doi: 10.1007/s10072-024-07485-w. Epub 2024 Apr 1. Neurol Sci. 2024. PMID: 38558319 Free PMC article.
-
Narrative Review of Brivaracetam: Preclinical Profile and Clinical Benefits in the Treatment of Patients with Epilepsy.Adv Ther. 2024 Jul;41(7):2682-2699. doi: 10.1007/s12325-024-02876-z. Epub 2024 May 29. Adv Ther. 2024. PMID: 38811492 Free PMC article. Review.
-
Post-marketing Experience with Cenobamate in the Treatment of Focal Epilepsies: A Multicentre Cohort Study.CNS Drugs. 2025 Mar;39(3):321-331. doi: 10.1007/s40263-025-01158-8. Epub 2025 Feb 15. CNS Drugs. 2025. PMID: 39954117 Free PMC article.
References
-
- Domínguez-Aguilera MC, Muñiz-Landeros CE. Prevalence of psychiatric disorders in patients with epilepsy in a tertiary level care hospital: Detection through the MINI PLUS International Structured Interview. Medicina Universitaria. 2017;19:3–6. doi: 10.1016/j.rmu.2016.11.003. - DOI

