Complication Rates of Central Venous Catheters: A Systematic Review and Meta-Analysis
- PMID: 38436976
- PMCID: PMC12285596
- DOI: 10.1001/jamainternmed.2023.8232
Complication Rates of Central Venous Catheters: A Systematic Review and Meta-Analysis
Erratum in
-
Errors in Supplement 1.JAMA Intern Med. 2024 Jun 1;184(6):707. doi: 10.1001/jamainternmed.2024.2175. JAMA Intern Med. 2024. PMID: 38767876 Free PMC article. No abstract available.
Abstract
Importance: Central venous catheters (CVCs) are commonly used but are associated with complications. Quantifying complication rates is essential for guiding CVC utilization decisions.
Objective: To summarize current rates of CVC-associated complications.
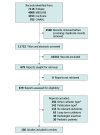
Data sources: MEDLINE, Embase, CINAHL, and CENTRAL databases were searched for observational studies and randomized clinical trials published between 2015 to 2023.
Study selection: This study included English-language observational studies and randomized clinical trials of adult patients that reported complication rates of short-term centrally inserted CVCs and data for 1 or more outcomes of interest. Studies that evaluated long-term intravascular devices, focused on dialysis catheters not typically used for medication administration, or studied catheters placed by radiologists were excluded.
Data extraction and synthesis: Two reviewers independently extracted data and assessed risk of bias. Bayesian random-effects meta-analysis was applied to summarize event rates. Rates of placement complications (events/1000 catheters with 95% credible interval [CrI]) and use complications (events/1000 catheter-days with 95% CrI) were estimated.
Main outcomes and measures: Ten prespecified complications associated with CVC placement (placement failure, arterial puncture, arterial cannulation, pneumothorax, bleeding events requiring action, nerve injury, arteriovenous fistula, cardiac tamponade, arrhythmia, and delay of ≥1 hour in vasopressor administration) and 5 prespecified complications associated with CVC use (malfunction, infection, deep vein thrombosis [DVT], thrombophlebitis, and venous stenosis) were assessed. The composite of 4 serious complications (arterial cannulation, pneumothorax, infection, or DVT) after CVC exposure for 3 days was also assessed.
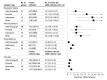
Results: Of 11 722 screened studies, 130 were included in the analyses. Seven of 15 prespecified complications were meta-analyzed. Placement failure occurred at 20.4 (95% CrI, 10.9-34.4) events per 1000 catheters placed. Other rates of CVC placement complications (per 1000 catheters) were arterial canulation (2.8; 95% CrI, 0.1-10), arterial puncture (16.2; 95% CrI, 11.5-22), and pneumothorax (4.4; 95% CrI, 2.7-6.5). Rates of CVC use complications (per 1000 catheter-days) were malfunction (5.5; 95% CrI, 0.6-38), infection (4.8; 95% CrI, 3.4-6.6), and DVT (2.7; 95% CrI, 1.0-6.2). It was estimated that 30.2 (95% CrI, 21.8-43.0) in 1000 patients with a CVC for 3 days would develop 1 or more serious complication (arterial cannulation, pneumothorax, infection, or DVT). Use of ultrasonography was associated with lower rates of arterial puncture (risk ratio [RR], 0.20; 95% CrI, 0.09-0.44; 13.5 events vs 68.8 events/1000 catheters) and pneumothorax (RR, 0.25; 95% CrI, 0.08-0.80; 2.4 events vs 9.9 events/1000 catheters).
Conclusions and relevance: Approximately 3% of CVC placements were associated with major complications. Use of ultrasonography guidance may reduce specific risks including arterial puncture and pneumothorax.
Conflict of interest statement
Figures


Comment in
-
Enhancing Quality and Safety in Critical Care-Challenges and Strategies for Central Venous Catheters.JAMA Intern Med. 2024 May 1;184(5):482-483. doi: 10.1001/jamainternmed.2023.8243. JAMA Intern Med. 2024. PMID: 38436978 No abstract available.
References
-
- iData Research. Central Venous Catheter Market Size, Share & Trends Analysis, Global, 2020-2026. 2020. Accessed October 26, 2023. https://idataresearch.com/product/vascular-access-devices-market/
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

