Chronic hepatitis B baseline viral load and on-treatment liver cancer risk: A multinational cohort study of HBeAg-positive patients
- PMID: 38436992
- PMCID: PMC11251501
- DOI: 10.1097/HEP.0000000000000752
Chronic hepatitis B baseline viral load and on-treatment liver cancer risk: A multinational cohort study of HBeAg-positive patients
Abstract
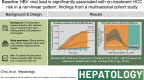
Background and aims: A single-nation study reported that pretreatment HBV viral load is associated with on-treatment risk of HCC in patients who are HBeAg-positive without cirrhosis and with chronic hepatitis B initiating antiviral treatment. We aimed to validate the association between baseline HBV viral load and on-treatment HCC risk in a larger, multinational cohort.
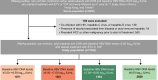
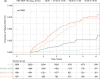
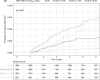
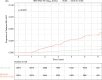
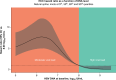
Approach and results: Using a multinational cohort from Korea, Hong Kong, and Taiwan involving 7545 adult patients with HBeAg-positive, without cirrhosis and with chronic hepatitis B who started entecavir or tenofovir treatment with baseline HBV viral load ≥5.00 log 10 IU/mL, HCC risk was estimated by baseline viral load. HBV viral load was analyzed as a categorical variable. During continuous antiviral treatment (median, 4.28 y), HCC developed in 200 patients (incidence rate, 0.61 per 100 person-years). Baseline HBV DNA level was independently associated with on-treatment HCC risk in a nonlinear pattern. HCC risk was lowest with the highest baseline viral load (≥8.00 log 10 IU/mL; incidence rate, 0.10 per 100 person-years), but increased sharply as baseline viral load decreased. The adjusted HCC risk was 8.05 times higher (95% CI, 3.34-19.35) with baseline viral load ≥6.00 and <7.00 log 10 IU/mL (incidence rate, 1.38 per 100 person-years) compared with high (≥8.00 log 10 IU/mL) baseline viral load ( p <0.001).
Conclusions: In a multinational cohort of adult patients with HBeAg-positive without cirrhosis and with chronic hepatitis B, baseline HBV viral load was significantly associated with HCC risk despite antiviral treatment. Patients with the highest viral load who initiated treatment had the lowest long-term risk of HCC development.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Terry Cheuk-Fung Yip consults, advises, is on the speakers’ bureau, and received grants from Gilead. W. Ray Kim advises Gilead, Inovio, and Roche. Leland J. Yee is employed by and owns stock in Gilead. Craig Brooks-Rooney is employed by Costello Medical. Tristan Curteis has other interests with Gilead. Laura J. Clark has other interests with Gilead. Zarena Jafry is employed by Costello Medical. Yi-Hsiang Huang consults, advises, and received grants from Gilead. He consults, advises, and is on the speakers’ bureau for Eisai and MSD. He advises and received grants from Bristol-Meyers Squibb. He advises AbbVie, AstraZeneca, Eli Lilly, Ipsen, Ono, and Roche. Cheng-Yuan Peng advises Bristol-Myers Squibb and Gilead. Grace Lai-Hung Wong advises, is on the speakers’ bureau for, and received grants from Gilead. She advises and is on the speakers’ bureau for Janssen. She advises AstraZeneca. She is on the speakers’ bureau for Abbott, AbbVie, Ascletis, Bristol-Myers Squibb, Echosens, Furui, and Roche. Young-Suk Lim advises and received grants from Gilead. The remaining authors have no conflicts to report.
Figures
Comment in
-
HCC risk in patients who are HBeAg-positive and have chronic hepatitis B under long-term nucleos(t)ide analogue therapy: New insights from Asia.Hepatology. 2024 Aug 1;80(2):260-262. doi: 10.1097/HEP.0000000000000796. Epub 2024 Feb 16. Hepatology. 2024. PMID: 38373094 No abstract available.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2021;71:209–249. - PubMed
-
- Lampertico P, Agarwal K, Berg T, Buti M, Janssen HLA, Papatheodoridis G, et al. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. - PubMed
-
- Cui F, Blach S, Manzengo Mingiedi C, Gonzalez MA, Sabry Alaama A, Mozalevskis A, et al. Global reporting of progress towards elimination of hepatitis B and hepatitis C. Lancet Gastroenterol Hepatol. 2023;8:332–342. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

