Batoclimab vs Placebo for Generalized Myasthenia Gravis: A Randomized Clinical Trial
- PMID: 38436998
- PMCID: PMC10913013
- DOI: 10.1001/jamaneurol.2024.0044
Batoclimab vs Placebo for Generalized Myasthenia Gravis: A Randomized Clinical Trial
Abstract
Importance: Myasthenia gravis (MG) is caused by autoantibodies that disrupt the neuromuscular junction. The neonatal fragment crystallizable receptor (FcRn) antagonists, efgartigimod and rozanolixizumab, reduce immunoglobulin G (IgG) level in the circulation and alleviate symptoms in patients with generalized MG.
Objective: To examine the efficacy and safety profile of batoclimab, a monoclonal IgG1 antibody, in patients with generalized MG.
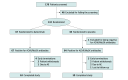
Design, setting, and participants: This was a multicenter randomized clinical trial conducted from September 15, 2021, to June 29, 2022, at 27 centers in China. Adult patients 18 years or older with generalized MG were screened, and those who were antibody positive were enrolled.
Intervention: Eligible patients received batoclimab or matching placebo in addition to standard of care. Each treatment cycle consisted of 6 weekly subcutaneous injections of batoclimab, 680 mg, or matching placebo followed by 4 weeks of observation. A second treatment cycle was conducted in patients who required continuing treatment.
Main outcome and measure: The primary outcome was sustained improvement, as defined by a 3-point or greater reduction in the Myasthenia Gravis Activities of Daily Living (MG-ADL) score from baseline for 4 or more consecutive weeks in the first cycle in individuals who were positive for acetylcholine receptor or muscle-specific kinase antibodies.
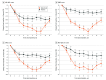
Results: A total of 178 adult patients with generalized MG were screened, 132 were randomly assigned, 131 tested positive for antibodies, and 1 tested negative for antibodies. A total of 132 patients (mean [SE] age, 43.8 [13.6] years; 88 women [67.2%]) were enrolled. The rate of sustained MG-ADL improvement in the first cycle in antibody-positive patients was 31.3% (20 of 64) in the placebo group vs 58.2% (39 of 67) in the batoclimab group (odds ratio, 3.45; 95% CI, 1.62-7.35; P = .001). The MG-ADL score diverged between the 2 groups as early as week 2. The mean (SE) maximum difference in MG-ADL score reduction occurred 1 week after the last dose (day 43, 1.7 [0.3] in the placebo group vs 3.6 [0.3] in the batoclimab group; group difference, -1.9; 95% CI, -2.8 to -1.0; nominal P < .001). The rates of treatment-related and severe treatment-emergent adverse events in patients were 36.9% (24 of 65) and 7.7% (5 of 65) in the placebo group vs 70.1% (47 of 67) and 3.0% (2 of 67) in the batoclimab group, respectively.
Conclusions and relevance: Batoclimab increased the rate of sustained MG-ADL improvement and was well tolerated in adult patients with generalized MG. Clinical effects and the extent of IgG reduction were similar to those previously reported for efgartigimod and rozanolixizumab. Future studies of large sample size are needed to further understand the safety profile of batoclimab.
Trial registration: ClinicalTrials.gov Identifier: NCT05039190.
Conflict of interest statement
Figures
References
Associated data
LinkOut - more resources
Full Text Sources
Medical

