Structure-guided design of VAR2CSA-based immunogens and a cocktail strategy for a placental malaria vaccine
- PMID: 38437239
- PMCID: PMC10939253
- DOI: 10.1371/journal.ppat.1011879
Structure-guided design of VAR2CSA-based immunogens and a cocktail strategy for a placental malaria vaccine
Abstract
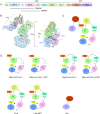
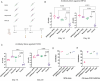
Placental accumulation of Plasmodium falciparum infected erythrocytes results in maternal anemia, low birth weight, and pregnancy loss. The parasite protein VAR2CSA facilitates the accumulation of infected erythrocytes in the placenta through interaction with the host receptor chondroitin sulfate A (CSA). Antibodies that prevent the VAR2CSA-CSA interaction correlate with protection from placental malaria, and VAR2CSA is a high-priority placental malaria vaccine antigen. Here, structure-guided design leveraging the full-length structures of VAR2CSA produced a stable immunogen that retains the critical conserved functional elements of VAR2CSA. The design expressed with a six-fold greater yield than the full-length protein and elicited antibodies that prevent adhesion of infected erythrocytes to CSA. The reduced size and adaptability of the designed immunogen enable efficient production of multiple variants of VAR2CSA for use in a cocktail vaccination strategy to increase the breadth of protection. These designs form strong foundations for the development of potent broadly protective placental malaria vaccines.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
NHT, RM and PED are inventors on a patent application related to this work. JYAD is an inventor on patent US9855321B2.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

