Stereotactic body radiation therapy for spinal metastases: A new standard of care
- PMID: 38437670
- PMCID: PMC10911798
- DOI: 10.1093/neuonc/noad225
Stereotactic body radiation therapy for spinal metastases: A new standard of care
Abstract
Advancements in systemic therapies for patients with metastatic cancer have improved overall survival and, hence, the number of patients living with spinal metastases. As a result, the need for more versatile and personalized treatments for spinal metastases to optimize long-term pain and local control has become increasingly important. Stereotactic body radiation therapy (SBRT) has been developed to meet this need by providing precise and conformal delivery of ablative high-dose-per-fraction radiation in few fractions while minimizing risk of toxicity. Additionally, advances in minimally invasive surgical techniques have also greatly improved care for patients with epidural disease and/or unstable spines, which may then be combined with SBRT for durable local control. In this review, we highlight the indications and controversies of SBRT along with new surgical techniques for the treatment of spinal metastases.
Keywords: SBRT; dose selection; local control; spinal metastases; target delineation.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
H.C.: research grant from Elekta, speaker’s honorarium from Novartis. A.S.: consultant for Varian, Elekta (Gamma Knife Icon), BrainLAB, Merck, Abbvie, Roche; Vice President of the International Stereotactic Radiosurgery Society (ISRS), Co-Chair of the AO Spine Knowledge Forum Tumor; received honorarium for past educational seminars for AstraZeneca, Elekta AB, Varian, BrainLAB, Accuray, Seagen Inc, research grant with Elekta AB, Varian, Seagen Inc, BrainLAB, and travel accommodations/expenses with Elekta, Varian and BrainLAB, belongs to the Elekta MR Linac Research Consortium and is a Clinical Steering Committee Member, and chairs the Elekta Oligometastases Group and the Elekta Gamma Knife Icon Group. C.B.: consultant for Depuy-Synthes, Bionaut Lab, Haystack Oncology, Galectin Therapeutics and Privo Technologies and co-founder of OrisDx and Belay Diagnostics. P.M.: none. K.J.R.: research fundings from Canon, Elekta AB and Accuray, data safety monitoring board BioMimetix, contract being reviewed for research funding from icotec, radiogenomics patent under development with Canon.
Figures
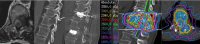

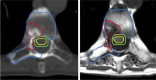
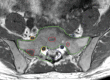
References
-
- Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643–648. - PubMed
-
- Fisher C, Ali Z, Detsky J, et al. Photodynamic therapy for the treatment of vertebral metastases: a phase I clinical trial. Clin Cancer Res. 2019;25(19):5766–5776. - PubMed
-
- Dunne EM, Liu MC, Lo SS, Sahgal A.. The changing landscape for the treatment of painful spinal metastases: is stereotactic body radiation therapy the new standard of care? Clin Oncol (R Coll Radiol). 2022;34(5):325–331. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

