CD19-CD28: an affinity-optimized CD28 agonist for combination with glofitamab (CD20-TCB) as off-the-shelf immunotherapy
- PMID: 38437725
- PMCID: PMC11143537
- DOI: 10.1182/blood.2023023381
CD19-CD28: an affinity-optimized CD28 agonist for combination with glofitamab (CD20-TCB) as off-the-shelf immunotherapy
Abstract
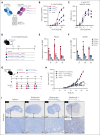
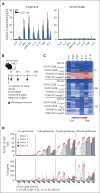
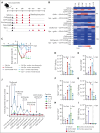
Effective T-cell responses not only require the engagement of T-cell receptors (TCRs; "signal 1"), but also the availability of costimulatory signals ("signal 2"). T-cell bispecific antibodies (TCBs) deliver a robust signal 1 by engaging the TCR signaling component CD3ε, while simultaneously binding to tumor antigens. The CD20-TCB glofitamab redirects T cells to CD20-expressing malignant B cells. Although glofitamab exhibits strong single-agent efficacy, adding costimulatory signaling may enhance the depth and durability of T-cell-mediated tumor cell killing. We developed a bispecific CD19-targeted CD28 agonist (CD19-CD28), RG6333, to enhance the efficacy of glofitamab and similar TCBs by delivering signal 2 to tumor-infiltrating T cells. CD19-CD28 distinguishes itself from the superagonistic antibody TGN1412, because its activity requires the simultaneous presence of a TCR signal and CD19 target binding. This is achieved through its engineered format incorporating a mutated Fc region with abolished FcγR and C1q binding, CD28 monovalency, and a moderate CD28 binding affinity. In combination with glofitamab, CD19-CD28 strongly increased T-cell effector functions in ex vivo assays using peripheral blood mononuclear cells and spleen samples derived from patients with lymphoma and enhanced glofitamab-mediated regression of aggressive lymphomas in humanized mice. Notably, the triple combination of glofitamab with CD19-CD28 with the costimulatory 4-1BB agonist, CD19-4-1BBL, offered substantially improved long-term tumor control over glofitamab monotherapy and respective duplet combinations. Our findings highlight CD19-CD28 as a safe and highly efficacious off-the-shelf combination partner for glofitamab, similar TCBs, and other costimulatory agonists. CD19-CD28 is currently in a phase 1 clinical trial in combination with glofitamab. This trial was registered at www.clinicaltrials.gov as #NCT05219513.
© 2024 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: J.S., T.H., C. Kuettel, C.C., S.H., G.G., K.K., M.L., M.J.E., D.M., C.J., K.L., S.M.K., B.G., J.M., S.K., L.K., S.W., S.B., S. Gebhardt, A.V., B.A., V.N., D.S., M. Bez, E.B., J.E., C.H., F.L., S.J., A.S., M.L.C., S.C., M. Bacac, S. Gasser, C. Klein, and P.U. are employees of Roche and hold ownership of Roche stocks and patents. The remaining authors declare no competing financial interests.
Figures




Comment in
-
Giving T-cell bispecifics a helping hand.Blood. 2024 May 23;143(21):2115-2116. doi: 10.1182/blood.2024024346. Blood. 2024. PMID: 38780922 No abstract available.
References
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

