Identification of distinct subgroups of Sjögren's disease by cluster analysis based on clinical and biological manifestations: data from the cross-sectional Paris-Saclay and the prospective ASSESS cohorts
- PMID: 38437852
- PMCID: PMC10949202
- DOI: 10.1016/S2665-9913(23)00340-5
Identification of distinct subgroups of Sjögren's disease by cluster analysis based on clinical and biological manifestations: data from the cross-sectional Paris-Saclay and the prospective ASSESS cohorts
Abstract
Background: Sjögren's disease is a heterogenous autoimmune disease with a wide range of symptoms-including dryness, fatigue, and pain-in addition to systemic manifestations and an increased risk of lymphoma. We aimed to identify distinct subgroups of the disease, using cluster analysis based on subjective symptoms and clinical and biological manifestations, and to compare the prognoses of patients in these subgroups.
Methods: This study included patients with Sjögren's disease from two independent cohorts in France: the cross-sectional Paris-Saclay cohort and the prospective Assessment of Systemic Signs and Evolution of Sjögren's Syndrome (ASSESS) cohort. We first used an unsupervised multiple correspondence analysis to identify clusters within the Paris-Saclay cohort using 26 variables comprising patient-reported symptoms and clinical and biological manifestations. Next, we validated these clusters using patients from the ASSESS cohort. Changes in disease activity (measured by the European Alliance of Associations for Rheumatology [EULAR] Sjögren's Syndrome Disease Activity Index [ESSDAI]), patient-acceptable symptom state (measured by the EULAR Sjögren's Syndrome Patient Reported Index [ESSPRI]), and lymphoma incidence during follow-up were compared between clusters. Finally, we compared our clusters with the symptom-based subgroups previously described by Tarn and colleagues.
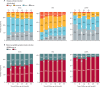
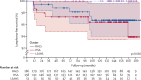
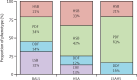
Findings: 534 patients from the Paris-Saclay cohort (502 [94%] women, 32 [6%] men, median age 54 years [IQR 43-64]), recruited between 1999 and 2022, and 395 patients from the ASSESS cohort (370 [94%] women, 25 [6%] men, median age 53 years [43-63]), recruited between 2006 and 2009, were included in this study. In both cohorts, hierarchical cluster analysis revealed three distinct subgroups of patients: those with B-cell active disease and low symptom burden (BALS), those with high systemic disease activity (HSA), and those with low systemic disease activity and high symptom burden (LSAHS). During follow-up in the ASSESS cohort, disease activity and symptom states worsened for patients in the BALS cluster (67 [36%] of 186 patients with ESSPRI score <5 at month 60 vs 92 [49%] of 186 at inclusion; p<0·0001). Lymphomas occurred in patients in the BALS cluster (five [3%] of 186 patients; diagnosed a median of 70 months [IQR 42-104] after inclusion) and the HSA cluster (six [4%] of 158 patients; diagnosed 23 months [13-83] after inclusion). All patients from the Paris-Saclay cohort with a history of lymphoma were in the BALS and HSA clusters. This unsupervised clustering classification based on symptoms and clinical and biological manifestations did not correlate with a previous classification based on symptoms only.
Interpretation: On the basis of symptoms and clinical and biological manifestations, we identified three distinct subgroups of patients with Sjögren's disease with different prognoses. Our results suggest that these subgroups represent different heterogeneous pathophysiological disease mechanisms, stages of disease, or both. These findings could be of interest when stratifying patients in future therapeutic trials.
Funding: Fondation pour la Recherche Médicale, French Ministry of Health, French Society of Rheumatology, Innovative Medicines Initiative 2 Joint Undertaking, Medical Research Council UK, and Foundation for Research in Rheumatology.
Copyright © 2024 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflicts of interest GN received honoraria from Boehringer and Novartis and travel fees from Amgen and AbbVie. W-FN received consulting fees from Resolve Therapeutics, Argenx, and Novartis and participated on data safety monitoring boards or advisory boards for Sanofi and Janssen Pharmaceuticals. JM received grants from Bristol Myers Squibb (BMS), Fresenius Kabi, Lilly, Novartis, Pfizer, and Roche-Chugai; received honoraria from AbbVie, Boehringer Ingelheim, Biogen, Lilly, Mylan, Pfizer, Sanofi, BMS, Fresenius Kabi, Galapagos, Medac, Novartis, and Roche-Chugai; received travel fees from BMS, Lilly, and Fresenius Kabi; and participated on advisory boards for AbbVie, Pfizer, and Galapagos. ED received consulting fees from BMS, Celgene, Lilly, Merck Sharp & Dohme (MSD), Novartis, and UCB; honoraria for lectures from AbbVie, BMS, Janssen Pharmaceuticals, Lilly, Medac, MSD, Novartis, Roche-Chugai, Sanofi, UCB, Celgene, Amgen, and Galapagos; and travel fees from AbbVie, BMS, Janssen Pharmaceuticals, Lilly, Medac, MSD, Novartis, Roche-Chugai, Sanofi, UCB, Celgene, Amgen, and Galapagos. PD received grants from Novartis and consulting fees from Pfizer, Roche-Chugai, BMS, AbbVie, and MSD. MC received travel fees from Janssen Pharmaceuticals, UCB, and Pfizer. CS received honoraria from Novartis and Roche-Chugai and travel fees from Novartis, Biogen, and Lilly. VLG received travel fees from AstraZeneca and Novartis. J-EG received grants from Pfizer, AbbVie, and Lilly and consulting fees from AbbVie, AstraZeneca, Sanofi, Lilly, Galapagos, Gilead Sciences, Roche-Chugai, Pfizer, BMS, and MSD. XM received consulting fees from AstraZeneca, BMS, Galapagos, GSK, Novartis, and Pfizer. RS received consulting fees from GSK, BMS, Boehringer Ingelheim, and Janssen Pharmaceuticals; honoraria from GSK, BMS, Boehringer Ingelheim, Amgen, Pfizer, and Roche-Chugai; and travel fees from Amgen and GSK. All other authors declare no competing interests.
Figures

References
-
- Mariette X, Criswell LA. Primary Sjögren's syndrome. N Engl J Med. 2018;378:931–939. - PubMed
-
- Mahr A, Katsahian S, Varet H, et al. Revisiting the classification of clinical phenotypes of anti-neutrophil cytoplasmic antibody-associated vasculitis: a cluster analysis. Ann Rheum Dis. 2013;72:1003–1010. - PubMed
-
- Dion J, Costedoat-Chalumeau N, Sène D, et al. Relapsing polychondritis can be characterized by three different clinical phenotypes: analysis of a recent series of 142 patients. Arthritis Rheumatol. 2016;68:2992–3001. - PubMed
-
- Font J, Cervera R, Ramos-Casals M, et al. Clusters of clinical and immunologic features in systemic lupus erythematosus: analysis of 600 patients from a single center. Semin Arthritis Rheum. 2004;33:217–230. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

