Could Less Be More? Accounting for Fractional-Dose Regimens and Different Number of Vaccine Doses When Measuring the Impact of the RTS,S/AS01E Malaria Vaccine
- PMID: 38438123
- PMCID: PMC11326831
- DOI: 10.1093/infdis/jiae075
Could Less Be More? Accounting for Fractional-Dose Regimens and Different Number of Vaccine Doses When Measuring the Impact of the RTS,S/AS01E Malaria Vaccine
Abstract
Background: The RTS,S/AS01E (RTS,S) malaria vaccine is recommended for children in malaria endemic areas. This phase 2b trial evaluates RTS,S fractional- and full-dose regimens in Ghana and Kenya.
Methods: In total, 1500 children aged 5-17 months were randomized (1:1:1:1:1) to receive RTS,S or rabies control vaccine. RTS,S groups received 2 full RTS,S doses at months 0 and 1 and either full (groups R012-20, R012-14-26) or fractional doses (one-fifth; groups Fx012-14-26, Fx017-20-32).
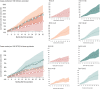
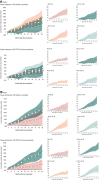
Results: At month 32 post-dose 1, vaccine efficacy against clinical malaria (all episodes) ranged from 38% (R012-20; 95% confidence interval [CI]: 24%-49%) to 53% (R012-14-26; 95% CI: 42%-62%). Vaccine impact (cumulative number of cases averted/1000 children vaccinated) was 1344 (R012-20), 2450 (R012-14-26), 2273 (Fx012-14-26), and 2112 (Fx017-20-32). To account for differences in vaccine volume (fractional vs full dose; post hoc analysis), we estimated cases averted/1000 RTS,S full-dose equivalents: 336 (R012-20), 490 (R012-14-26), 874 (Fx012-14-26), and 880 (Fx017-20-32).
Conclusions: Vaccine efficacy was similar across RTS,S groups. Vaccine impact accounting for full-dose equivalence suggests that using fractional-dose regimens could be a viable dose-sparing strategy. If maintained through trial end, these observations underscore the means to reduce cost per regimen thus maximizing impact and optimizing supply.
Clinical trials registration: NCT03276962 (ClinicalTrials.gov).
Keywords: RTS, S/AS01E; fractional-dose regimen; malaria vaccine; vaccine efficacy; vaccine impact.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. L. S., A. Bo., M. L., F. R., and O. O.-A. are GSK employees. L. S., F. R., and O. O.-A. have restricted shares in GSK. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

Similar articles
-
Efficacy of RTS,S/AS01E malaria vaccine administered according to different full, fractional, and delayed third or early fourth dose regimens in children aged 5-17 months in Ghana and Kenya: an open-label, phase 2b, randomised controlled trial.Lancet Infect Dis. 2022 Sep;22(9):1329-1342. doi: 10.1016/S1473-3099(22)00273-0. Epub 2022 Jun 23. Lancet Infect Dis. 2022. PMID: 35753316 Free PMC article. Clinical Trial.
-
Genotypic analysis of RTS,S/AS01E malaria vaccine efficacy against parasite infection as a function of dosage regimen and baseline malaria infection status in children aged 5-17 months in Ghana and Kenya: a longitudinal phase 2b randomised controlled trial.Lancet Infect Dis. 2024 Sep;24(9):1025-1036. doi: 10.1016/S1473-3099(24)00179-8. Epub 2024 May 6. Lancet Infect Dis. 2024. PMID: 38723650 Free PMC article. Clinical Trial.
-
A Phase IIa Controlled Human Malaria Infection and Immunogenicity Study of RTS,S/AS01E and RTS,S/AS01B Delayed Fractional Dose Regimens in Malaria-Naive Adults.J Infect Dis. 2020 Oct 13;222(10):1681-1691. doi: 10.1093/infdis/jiaa421. J Infect Dis. 2020. PMID: 32687161 Free PMC article. Clinical Trial.
-
RTS,S: Toward a first landmark on the Malaria Vaccine Technology Roadmap.Vaccine. 2015 Dec 22;33(52):7425-32. doi: 10.1016/j.vaccine.2015.09.061. Epub 2015 Oct 1. Vaccine. 2015. PMID: 26431982 Review.
-
The RTS,S/AS01 malaria vaccine in children 5 to 17 months of age at first vaccination.Expert Rev Vaccines. 2016 Dec;15(12):1481-1493. doi: 10.1080/14760584.2016.1236689. Expert Rev Vaccines. 2016. PMID: 27841689 Review.
References
-
- World Health Organization . World malaria report 2022. Available at: https://www.who.int/teams/global-malaria-programme/reports/world-malaria.... Accessed 8 August 2023.
-
- World Health Organization . Malaria vaccine: WHO position paper—March 2022. Wkly Epidemiol Rec 2022; 97:61–80.
-
- World Health Organization . WHO recommends R21/Matrix-M vaccine for malaria prevention in updated advice on immunization. Available at: http://www.who.int/news/item/02-10-2023-who-recommends-r21-matrix-m-vacc.... Accessed 8 January 2024.
-
- Chandramohan D, Zongo I, Sagara I, et al. . Seasonal malaria vaccination with or without seasonal malaria chemoprevention. N Engl J Med 2021; 385:1005–17. - PubMed
-
- World Health Organization . WHO position on the use of fractional doses—June 2017, addendum to vaccines and vaccination against yellow fever WHO: position paper—June 2013. Vaccine 2017; 35:5751–2. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

