Disposables used cumulatively in routine IVF procedures could display toxicity
- PMID: 38438162
- PMCID: PMC11063546
- DOI: 10.1093/humrep/deae028
Disposables used cumulatively in routine IVF procedures could display toxicity
Abstract
Study question: Is there a cumulative toxicity of disposables used in IVF procedures?
Summary answer: A toxicity may be detected when consumables are used cumulatively, while no toxicity is detected when the same consumables are used and tested individually.
What is known already: Many components of items used in IVF laboratories may impair human embryonic development. Consequently, it is necessary to screen all reagents and materials which could be in contact with gametes and embryos. Toxicity tests, such as the mouse embryo assay and the human sperm motility assay (HSMA), are used by manufacturers as quality control tools to demonstrate the safety of their products. This evaluation is currently individually performed for each single consumable. However, during an IVF cycle, several devices are used sequentially, potentially creating a cumulative exposure to chemical contaminants, which could not be detected for individually tested consumables.
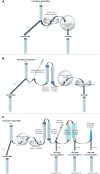
Study design, size, duration: The objective of this observational study conducted from March 2021 to October 2022 was to evaluate with the HSMA methodology if there was a cumulative toxicity when several disposables are sequentially used. Fourteen categories of consumables currently used in routine IVF procedures were studied, which included devices used for sperm and oocyte collection (cups, condoms, and oocyte aspiration needles), manipulation (flasks, tubes, tips, pipettes, embryo transfer catheters, syringes, and gloves), culture (dishes), and storage (straws).
Participants/materials, setting, methods: After obtaining patient consent, the surplus semen assessed as having normal parameters according to the World Health Organization 2010 criteria were used to perform the HSMAs. First, each consumable was tested individually. Then, associations of three, four, and five consumables, previously validated as non-toxic when tested individually, were analyzed. HSMAs were conducted three times to ensure reproducibility, with a defined toxicity threshold of a sperm motility index (SMI) below 0.85 in at least two of three tests.
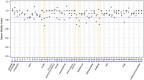
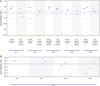
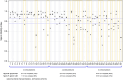
Main results and the role of chance: Thirty-six references of disposables were first individually tested across 53 lots. Forty-nine (92%) demonstrated compliance. However, four (8%) devices revealed toxicity: one lot of 1 ml syringes, two lots of sperm cups, and one lot of 25 cm2 flasks. These four references were excluded from the IVF routine procedures. A total of 48 combinations of consumables were assessed, involving 41 lots from 32 references that were previously individually tested. Among the evaluated combinations, 17 out of 48 (35%) associations exhibited toxicity with a SMI below 0.85 for two of the three tests (n = 8) or all the three tests (n = 9). Notably, three out of 17 (18%) of the three-consumable associations, five out of 16 (31%) of the four-consumable associations, and nine out of 15 (60%) of the five-consumable associations were found not compliant. The toxicity did not originate from a single consumable, because only consumables that were individually pre-validated as non-toxic were included in the combinations, but the toxicity had a cumulative origin. The risk of cumulative toxicity increased with the number of consumables included in the association (Cochran-Mantel-Haenszel statistic, P = 0.013).
Limitations, reasons for caution: The high proportion of non-compliant combinations of disposables can be attributed directly to the extreme rigorous extraction conditions employed during the tests, which could deviate from the conditions encountered in routine clinical use. Also, the methodology employed in the HSMAs (e.g. toxicity extraction duration, sperm concentrations, and protein supplementation of the medium) can influence the sensitivity of the tests.
Wider implications of the findings: This study highlights the significance of performing toxicity testing on devices before introducing them into clinical practice. Disposables should be tested individually to detect immediate toxicities and also in combination. Our results advocate rationalizing the number of consumables used in each IVF procedure and re-evaluating the use of glass consumables.
Study funding/competing interest(s): This study received fundings from GCS Ramsay Santé pour l'Enseignement et la Recherche (Paris, France) and the Centre de Biologie Médicale BIOGROUP (Le Chesnay-Rocquencourt, France). The authors declare that they have no conflict of interest that could be perceived as prejudicing the impartiality of the reported research.
Trial registration number: N/A.
Keywords: IVF; cumulative; disposable devices; human sperm motility assay; plastics; toxicity.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
The authors declare that they have no conflict of interest that could be perceived as prejudicing the impartiality of the reported research.
Figures
Similar articles
-
Embryotoxicity testing of IVF disposables: how do manufacturers test?Hum Reprod. 2020 Feb 29;35(2):283-292. doi: 10.1093/humrep/dez277. Hum Reprod. 2020. PMID: 32053198
-
Antioxidants improve IVF outcome and subsequent embryo development in the mouse.Hum Reprod. 2017 Dec 1;32(12):2404-2413. doi: 10.1093/humrep/dex330. Hum Reprod. 2017. PMID: 29136144
-
Spermaurin, an La1-like peptide from the venom of the scorpion Scorpio maurus palmatus, improves sperm motility and fertilization in different mammalian species.Mol Hum Reprod. 2017 Feb 10;23(2):116-131. doi: 10.1093/molehr/gaw075. Mol Hum Reprod. 2017. PMID: 27932550
-
Revised guidelines for good practice in IVF laboratories (2015).Hum Reprod. 2016 Apr;31(4):685-6. doi: 10.1093/humrep/dew016. Epub 2016 Feb 17. Hum Reprod. 2016. PMID: 26908842 Review.
-
Retrospective analysis: reproducibility of interblastomere differences of mRNA expression in 2-cell stage mouse embryos is remarkably poor due to combinatorial mechanisms of blastomere diversification.Mol Hum Reprod. 2018 Jul 1;24(7):388-400. doi: 10.1093/molehr/gay021. Mol Hum Reprod. 2018. PMID: 29746690
Cited by
-
Effect of Liquid Marble 3D Culture System on In Vitro Maturation and Embryo Development of Prepubertal Goat Oocytes.Animals (Basel). 2025 Jan 12;15(2):188. doi: 10.3390/ani15020188. Animals (Basel). 2025. PMID: 39858188 Free PMC article.
References
-
- Ackerman SB, Stokes GL, Swanson RJ, Taylor SP, Fenwick L.. Toxicity testing for human in vitro fertilization programs. J In Vitro Fert Embryo Transf 1985;2:132–137. - PubMed
-
- Afreen V, Hashmi K, Nasir R, Saleem A, Khan MI, Akhtar MF.. Adverse health effects and mechanisms of microplastics on female reproductive system: a descriptive review. Environ Sci Pollut Res Int 2023;30:76283–76296. - PubMed
-
- Björndahl L, Barratt CLR, Mortimer D, Jouannet P.. “How to count sperm properly”: checklist for acceptability of studies based on human semen analysis. Hum Reprod 2016;31:227–232. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

