NDUFS4 regulates cristae remodeling in diabetic kidney disease
- PMID: 38438382
- PMCID: PMC10912198
- DOI: 10.1038/s41467-024-46366-w
NDUFS4 regulates cristae remodeling in diabetic kidney disease
Erratum in
-
Publisher Correction: NDUFS4 regulates cristae remodeling in diabetic kidney disease.Nat Commun. 2024 Apr 8;15(1):3022. doi: 10.1038/s41467-024-47414-1. Nat Commun. 2024. PMID: 38589378 Free PMC article. No abstract available.
Abstract
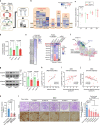
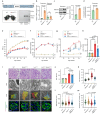
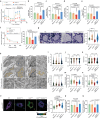
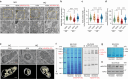
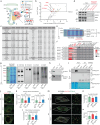
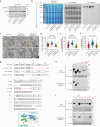
The mitochondrial electron transport chain (ETC) is a highly adaptive process to meet metabolic demands of the cell, and its dysregulation has been associated with diverse clinical pathologies. However, the role and nature of impaired ETC in kidney diseases remains poorly understood. Here, we generate diabetic mice with podocyte-specific overexpression of Ndufs4, an accessory subunit of mitochondrial complex I, as a model investigate the role of ETC integrity in diabetic kidney disease (DKD). We find that conditional male mice with genetic overexpression of Ndufs4 exhibit significant improvements in cristae morphology, mitochondrial dynamics, and albuminuria. By coupling proximity labeling with super-resolution imaging, we also identify the role of cristae shaping protein STOML2 in linking NDUFS4 with improved cristae morphology. Together, we provide the evidence on the central role of NDUFS4 as a regulator of cristae remodeling and mitochondrial function in kidney podocytes. We propose that targeting NDUFS4 represents a promising approach to slow the progression of DKD.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Update of
-
NDUFS4 Regulates Cristae Remodeling in Diabetic Kidney Disease.Res Sq [Preprint]. 2023 Jun 30:rs.3.rs-3070079. doi: 10.21203/rs.3.rs-3070079/v1. Res Sq. 2023. Update in: Nat Commun. 2024 Mar 4;15(1):1965. doi: 10.1038/s41467-024-46366-w. PMID: 37461606 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

