NRF2 activation ameliorates blood-brain barrier injury after cerebral ischemic stroke by regulating ferroptosis and inflammation
- PMID: 38438409
- PMCID: PMC10912757
- DOI: 10.1038/s41598-024-53836-0
NRF2 activation ameliorates blood-brain barrier injury after cerebral ischemic stroke by regulating ferroptosis and inflammation
Abstract
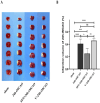
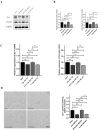
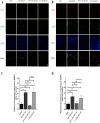
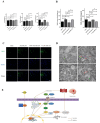
Arterial occlusion-induced ischemic stroke (IS) is a highly frequent stroke subtype. Nuclear factor erythroid 2-related factor 2 (NRF2) is a transcription factor that modulates antioxidant genes. Its role in IS is still unelucidated. The current study focused on constructing a transient middle cerebral artery occlusion (tMCAO) model for investigating the NRF2-related mechanism underlying cerebral ischemia/reperfusion (I/R) injury. Each male C57BL/6 mouse was injected with/with no specific NRF2 activator post-tMCAO. Changes in blood-brain barrier (BBB)-associated molecule levels were analyzed using western-blotting, PCR, immunohistochemistry, and immunofluorescence analysis. NRF2 levels within cerebral I/R model decreased at 24-h post-ischemia. NRF2 activation improved brain edema, infarct volume, and neurological deficits after MCAO/R. Similarly, sulforaphane (SFN) prevented the down-regulated tight junction proteins occludin and zonula occludens 1 (ZO-1) and reduced the up-regulated aquaporin 4 (AQP4) and matrix metalloproteinase 9 (MMP9) after tMCAO. Collectively, NRF2 exerted a critical effect on preserving BBB integrity modulating ferroptosis and inflammation. Because NRF2 is related to BBB injury regulation following cerebral I/R, this provides a potential therapeutic target and throws light on the underlying mechanism for clinically treating IS.
Keywords: Blood–brain barrier; Ferroptosis; Inflammation; Ischemic stroke; NRF2.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

