Elevation of astrocyte-derived extracellular vesicles over the first month post-stroke in humans
- PMID: 38438491
- PMCID: PMC10912590
- DOI: 10.1038/s41598-024-55983-w
Elevation of astrocyte-derived extracellular vesicles over the first month post-stroke in humans
Abstract
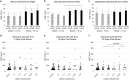
We sought to identify alterations in the quantity of plasma brain-derived extracellular vesicles (EV) over the first month post-stroke to shed light on related injury and repair mechanisms. We assessed plasma levels of presumed neuron-derived EVs (NDEs), astrocyte-derived EVs (ADEs), and oligodendrocyte-derived EVs (ODEs) in 58 patients 5, 15, and 30 days post-ischemic stroke and 46 controls matched for cardiovascular risk factors using sandwich immunoassays. Subsets of brain-derived EVs were identified by co-expression of the general EV marker CD9 and markers for neurons (L1CAM, CD171), astrocytes (EAAT1), and oligodendrocytes (MOG) respectively. Clinical MRIs assessed lesion volume and presence of hemorrhagic transformation. ADE levels were elevated 5, 15, and 30 days post-stroke compared to controls (p = 0.002, p = 0.002, and p = 0.005 respectively) with no significant change for NDE or ODE. ADEs were increased 15 days post-stroke in patients with hemorrhagic transformation (p = 0.04) compared to patients with no hemorrhage. We conclude that ADE levels are preferentially increased over the first month post-stroke in humans, possibly to provide trophic support to injured neurons following ischemia. ADEs hold potential as biomarkers of blood-brain barrier breakdown and hemorrhagic transformation, but this requires further study at earlier time points post-stroke.
Keywords: Astrocytes; Excitatory amino acid transporter 1; Exosomes; Extracellular vesicles; Hemorrhagic stroke; Ischemic stroke; Myelin oligodendrocyte glycoprotein; Neural cell adhesion molecule L1.
© 2024. The Author(s).
Conflict of interest statement
The study sponsor, NanoSomiX, Inc., provided support in the form of salaries for authors D.V. and M.M., and half the funding to isolate and quantify EV populations from plasma samples, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. NanoSomiX holds issued US and International pending patents on the methods for capture and analysis of plasma EVs used in this study. Georgetown University and NanoSomiX, Inc. have a collaborative agreement in place and have filed a provisional patent related to the use of brain-derived EVs in the care and diagnosis of stroke patients with M.A.E, M.M. and D.V. named as inventors. M.A.E. declares no other competing interests. This work was supported by internal funds from Georgetown University (M.A.E.) and support to analyze plasma samples was provided by NanoSomiX, Inc.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

