α4 nicotinic receptors on GABAergic neurons mediate a cholinergic analgesic circuit in the substantia nigra pars reticulata
- PMID: 38438581
- PMCID: PMC11130268
- DOI: 10.1038/s41401-024-01234-7
α4 nicotinic receptors on GABAergic neurons mediate a cholinergic analgesic circuit in the substantia nigra pars reticulata
Abstract
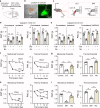
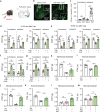
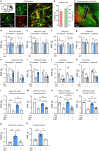
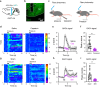
Nicotinic acetylcholine receptors (nAChRs) regulate pain pathways with various outcomes depending on receptor subtypes, neuron types, and locations. But it remains unknown whether α4β2 nAChRs abundantly expressed in the substantia nigra pars reticulata (SNr) have potential to mitigate hyperalgesia in pain states. We observed that injection of nAChR antagonists into the SNr reduced pain thresholds in naïve mice, whereas injection of nAChR agonists into the SNr relieved hyperalgesia in mice, subjected to capsaicin injection into the lower hind leg, spinal nerve injury, chronic constriction injury, or chronic nicotine exposure. The analgesic effects of nAChR agonists were mimicked by optogenetic stimulation of cholinergic inputs from the pedunculopontine nucleus (PPN) to the SNr, but attenuated upon downregulation of α4 nAChRs on SNr GABAergic neurons and injection of dihydro-β-erythroidine into the SNr. Chronic nicotine-induced hyperalgesia depended on α4 nAChRs in SNr GABAergic neurons and was associated with the reduction of ACh release in the SNr. Either activation of α4 nAChRs in the SNr or optogenetic stimulation of the PPN-SNr cholinergic projection mitigated chronic nicotine-induced hyperalgesia. Interestingly, mechanical stimulation-induced ACh release was significantly attenuated in mice subjected to either capsaicin injection into the lower hind leg or SNI. These results suggest that α4 nAChRs on GABAergic neurons mediate a cholinergic analgesic circuit in the SNr, and these receptors may be effective therapeutic targets to relieve hyperalgesia in acute and chronic pain, and chronic nicotine exposure.
Keywords: cholinergic system; chronic nicotine; nicotinic acetylcholine receptor; pain; pedunculopontine nucleus; substantia nigra pars reticulata.
© 2024. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
A role for α4(non-α6)* nicotinic acetylcholine receptors in motor behavior.Neuropharmacology. 2013 Oct;73:19-30. doi: 10.1016/j.neuropharm.2013.05.001. Epub 2013 May 17. Neuropharmacology. 2013. PMID: 23688922 Free PMC article.
-
Functional Upregulation of α4* Nicotinic Acetylcholine Receptors in VTA GABAergic Neurons Increases Sensitivity to Nicotine Reward.J Neurosci. 2015 Jun 3;35(22):8570-8. doi: 10.1523/JNEUROSCI.4453-14.2015. J Neurosci. 2015. PMID: 26041923 Free PMC article.
-
Mice lacking α4 nicotinic acetylcholine receptors are protected against alcohol-associated liver injury.Alcohol Clin Exp Res. 2022 Aug;46(8):1371-1383. doi: 10.1111/acer.14893. Epub 2022 Jul 3. Alcohol Clin Exp Res. 2022. PMID: 35723023 Free PMC article.
-
Interventions to reduce harm from continued tobacco use.Cochrane Database Syst Rev. 2016 Oct 13;10(10):CD005231. doi: 10.1002/14651858.CD005231.pub3. Cochrane Database Syst Rev. 2016. PMID: 27734465 Free PMC article.
-
Interventions for preventing weight gain after smoking cessation.Cochrane Database Syst Rev. 2012 Jan 18;1:CD006219. doi: 10.1002/14651858.CD006219.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2021 Oct 6;10:CD006219. doi: 10.1002/14651858.CD006219.pub4. PMID: 22258966 Updated.
Cited by
-
Activation of Pedunculopontine Tegmental Nucleus Alleviates the Pain Induced by the Lesion of Midbrain Dopaminergic Neurons.Int J Mol Sci. 2024 May 22;25(11):5636. doi: 10.3390/ijms25115636. Int J Mol Sci. 2024. PMID: 38891832 Free PMC article.
References
-
- Noori A, Sadeghirad B, Wang L, Siemieniuk RAC, Shokoohi M, Kum E, et al. Comparative benefits and harms of individual opioids for chronic non-cancer pain: a systematic review and network meta-analysis of randomised trials. Br J Anaesth. 2022;129:394–406. doi: 10.1016/j.bja.2022.05.031. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

