LRRK2 kinase inhibition reverses G2019S mutation-dependent effects on tau pathology progression
- PMID: 38438877
- PMCID: PMC10910783
- DOI: 10.1186/s40035-024-00403-2
LRRK2 kinase inhibition reverses G2019S mutation-dependent effects on tau pathology progression
Abstract
Background: Mutations in leucine-rich repeat kinase 2 (LRRK2) are the most common cause of familial Parkinson's disease (PD). These mutations elevate the LRRK2 kinase activity, making LRRK2 kinase inhibitors an attractive therapeutic. LRRK2 kinase activity has been consistently linked to specific cell signaling pathways, mostly related to organelle trafficking and homeostasis, but its relationship to PD pathogenesis has been more difficult to define. LRRK2-PD patients consistently present with loss of dopaminergic neurons in the substantia nigra but show variable development of Lewy body or tau tangle pathology. Animal models carrying LRRK2 mutations do not develop robust PD-related phenotypes spontaneously, hampering the assessment of the efficacy of LRRK2 inhibitors against disease processes. We hypothesized that mutations in LRRK2 may not be directly related to a single disease pathway, but instead may elevate the susceptibility to multiple disease processes, depending on the disease trigger. To test this hypothesis, we have previously evaluated progression of α-synuclein and tau pathologies following injection of proteopathic seeds. We demonstrated that transgenic mice overexpressing mutant LRRK2 show alterations in the brain-wide progression of pathology, especially at older ages.
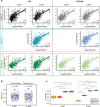
Methods: Here, we assess tau pathology progression in relation to long-term LRRK2 kinase inhibition. Wild-type or LRRK2G2019S knock-in mice were injected with tau fibrils and treated with control diet or diet containing LRRK2 kinase inhibitor MLi-2 targeting the IC50 or IC90 of LRRK2 for 3-6 months. Mice were evaluated for tau pathology by brain-wide quantitative pathology in 844 brain regions and subsequent linear diffusion modeling of progression.
Results: Consistent with our previous work, we found systemic alterations in the progression of tau pathology in LRRK2G2019S mice, which were most pronounced at 6 months. Importantly, LRRK2 kinase inhibition reversed these effects in LRRK2G2019S mice, but had minimal effect in wild-type mice, suggesting that LRRK2 kinase inhibition is likely to reverse specific disease processes in G2019S mutation carriers. Additional work may be necessary to determine the potential effect in non-carriers.
Conclusions: This work supports a protective role of LRRK2 kinase inhibition in G2019S carriers and provides a rational workflow for systematic evaluation of brain-wide phenotypes in therapeutic development.
Keywords: Cell-to-cell spread; G2019S; Genetic risk; MLi-2; Mapt; Transmission.
© 2024. The Author(s).
Conflict of interest statement
C.E.G.L. and M.J.F. are salaried employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

