Inhibition of Drp1- Fis1 interaction alleviates aberrant mitochondrial fragmentation and acute kidney injury
- PMID: 38439028
- PMCID: PMC10910703
- DOI: 10.1186/s11658-024-00553-1
Inhibition of Drp1- Fis1 interaction alleviates aberrant mitochondrial fragmentation and acute kidney injury
Abstract
Background: Acute kidney injury (AKI) is a common clinical disorder with complex etiology and poor prognosis, and currently lacks specific and effective treatment options. Mitochondrial dynamics dysfunction is a prominent feature in AKI, and modulation of mitochondrial morphology may serve as a potential therapeutic approach for AKI.
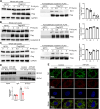
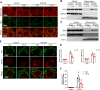
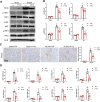
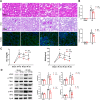
Methods: We induced ischemia-reperfusion injury (IRI) in mice (bilateral) and Bama pigs (unilateral) by occluding the renal arteries. ATP depletion and recovery (ATP-DR) was performed on proximal renal tubular cells to simulate in vitro IRI. Renal function was evaluated using creatinine and urea nitrogen levels, while renal structural damage was assessed through histopathological staining. The role of Drp1 was investigated using immunoblotting, immunohistochemistry, immunofluorescence, and immunoprecipitation techniques. Mitochondrial morphology was evaluated using confocal microscopy.
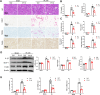
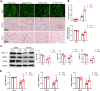
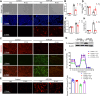
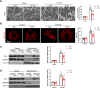
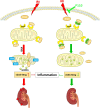
Results: Renal IRI induced significant mitochondrial fragmentation, accompanied by Dynamin-related protein 1 (Drp1) translocation to the mitochondria and Drp1 phosphorylation at Ser616 in the early stages (30 min after reperfusion), when there was no apparent structural damage to the kidney. The use of the Drp1 inhibitor P110 significantly improved kidney function and structural damage. P110 reduced Drp1 mitochondrial translocation, disrupted the interaction between Drp1 and Fis1, without affecting the binding of Drp1 to other mitochondrial receptors such as MFF and Mid51. High-dose administration had no apparent toxic side effects. Furthermore, ATP-DR induced mitochondrial fission in renal tubular cells, accompanied by a decrease in mitochondrial membrane potential and an increase in the translocation of the pro-apoptotic protein Bax. This process facilitated the release of dsDNA, triggering the activation of the cGAS-STING pathway and promoting inflammation. P110 attenuated mitochondrial fission, suppressed Bax mitochondrial translocation, prevented dsDNA release, and reduced the activation of the cGAS-STING pathway. Furthermore, these protective effects of P110 were also observed renal IRI model in the Bama pig and folic acid-induced nephropathy in mice.
Conclusions: Dysfunction of mitochondrial dynamics mediated by Drp1 contributes to renal IRI. The specific inhibitor of Drp1, P110, demonstrated protective effects in both in vivo and in vitro models of AKI.
Keywords: Acute kidney injury; Drp1; Fis1; Ischemia reperfusion injury; Mitochondria.
© 2024. The Author(s).
Conflict of interest statement
All the authors declared no competing interests.
Figures

References
-
- Acute kidney injury. Nat Rev Dis Primers. 2021;7(1):51. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

