Large-scale phosphoproteomics reveals activation of the MAPK/GADD45β/P38 axis and cell cycle inhibition in response to BMP9 and BMP10 stimulation in endothelial cells
- PMID: 38439036
- PMCID: PMC10910747
- DOI: 10.1186/s12964-024-01486-0
Large-scale phosphoproteomics reveals activation of the MAPK/GADD45β/P38 axis and cell cycle inhibition in response to BMP9 and BMP10 stimulation in endothelial cells
Abstract
Background: BMP9 and BMP10 are two major regulators of vascular homeostasis. These two ligands bind with high affinity to the endothelial type I kinase receptor ALK1, together with a type II receptor, leading to the direct phosphorylation of the SMAD transcription factors. Apart from this canonical pathway, little is known. Interestingly, mutations in this signaling pathway have been identified in two rare cardiovascular diseases, hereditary hemorrhagic telangiectasia and pulmonary arterial hypertension.
Methods: To get an overview of the signaling pathways modulated by BMP9 and BMP10 stimulation in endothelial cells, we employed an unbiased phosphoproteomic-based strategy. Identified phosphosites were validated by western blot analysis and regulated targets by RT-qPCR. Cell cycle analysis was analyzed by flow cytometry.
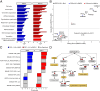
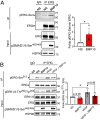
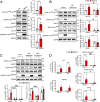
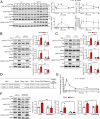
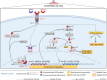
Results: Large-scale phosphoproteomics revealed that BMP9 and BMP10 treatment induced a very similar phosphoproteomic profile. These BMPs activated a non-canonical transcriptional SMAD-dependent MAPK pathway (MEKK4/P38). We were able to validate this signaling pathway and demonstrated that this activation required the expression of the protein GADD45β. In turn, activated P38 phosphorylated the heat shock protein HSP27 and the endocytosis protein Eps15 (EGF receptor pathway substrate), and regulated the expression of specific genes (E-selectin, hyaluronan synthase 2 and cyclooxygenase 2). This study also highlighted the modulation in phosphorylation of proteins involved in transcriptional regulation (phosphorylation of the endothelial transcription factor ERG) and cell cycle inhibition (CDK4/6 pathway). Accordingly, we found that BMP10 induced a G1 cell cycle arrest and inhibited the mRNA expression of E2F2, cyclinD1 and cyclinA1.
Conclusions: Overall, our phosphoproteomic screen identified numerous proteins whose phosphorylation state is impacted by BMP9 and BMP10 treatment, paving the way for a better understanding of the molecular mechanisms regulated by BMP signaling in vascular diseases.
Keywords: BMP10; BMP9; Endothelial cells; MAPK; Phosphoproteomics; Proliferation; Signaling.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

