LanGui tea, an herbal medicine formula, protects against binge alcohol-induced acute liver injury by activating AMPK-NLRP3 signaling
- PMID: 38439080
- PMCID: PMC10910869
- DOI: 10.1186/s13020-024-00906-0
LanGui tea, an herbal medicine formula, protects against binge alcohol-induced acute liver injury by activating AMPK-NLRP3 signaling
Abstract
Background: LanGui tea, a traditional Chinese medicine formulation comprising of Gynostemma pentaphyllum (Thunb.) Makino, Cinnamomum cassia (L.) J. Presl, and Ampelopsis grossedentata (Hand-Mazz) W.T. Wang, has yet to have its potential contributions to alcoholic liver disease (ALD) fully elucidated. Consequently, the objective of this research is to investigate the protective properties of LanGui tea against binge alcohol-induced ALD and the mechanisms underlying its effects.
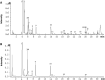
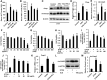
Methods: An experimental model of acute alcohol-induced liver disease was performed to assess the protective effects of extract of LanGui tea (ELG) at both 50 and 100 mg.kg-1 dosages on male C57BL/6 mice. Various parameters, including hepatic histological changes, inflammation, lipids content, as well as liver enzymes and interleukin 1β (IL-1β) in the serum were measured. The pharmacological mechanisms of ELG, specifically its effects on adenosine monophosphate-(AMP)-activated protein kinase (AMPK) and NLR family pyrin domain containing 3 (NLRP3) signaling, were investigated through Western blotting, qRT-PCR, ELISA, immunohistochemistry, immunofluorescence analyses, and by blocking the AMPK activity.
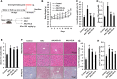
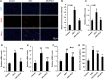
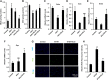
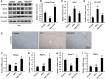
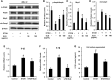
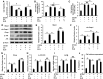
Results: ELG demonstrated a mitigating effect on fatty liver, inflammation, and hepatic dysfunction within the mouse model. This effect was achieved by activating AMPK signaling and inhibitingNLRP3 signaling in the liver, causing a reduction in IL-1β generation. In vitro studies further confirmed that ELG inhibited cell damage and IL-1β production in ethanol-induced hepatocytes by enhancing AMPK-NLRP3 signaling. Conversely, the pharmacological inhibition of AMPK activity nearly abrogated such alteration.
Conclusions: Thus, LanGui tea emerges as a promising herbal therapy for ALD management involving AMPK-NLRP3 signaling.
Keywords: Adenosine monophosphate-(AMP)-activated protein kinase; Alcoholic liver disease; Fatty liver; LanGui tea; NLR family pyrin domain containing 3.
© 2024. The Author(s).
Conflict of interest statement
No potential competing interests were reported.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

