A Dual-Gene Reporter-Amplifier Architecture for Enhancing the Sensitivity of Molecular MRI by Water Exchange
- PMID: 38439618
- PMCID: PMC11604348
- DOI: 10.1002/cbic.202400087
A Dual-Gene Reporter-Amplifier Architecture for Enhancing the Sensitivity of Molecular MRI by Water Exchange
Abstract
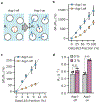
The development of genetic reporters for magnetic resonance imaging (MRI) is essential for investigating biological functions in vivo. However, current MRI reporters have low sensitivity, making it challenging to create significant contrast against the tissue background, especially when only a small fraction of cells express the reporter. To overcome this limitation, we developed an approach for amplifying the sensitivity of molecular MRI by combining a chemogenetic contrast mechanism with a biophysical approach to increase water diffusion through the co-expression of a dual-gene construct comprising an organic anion transporting polypeptide, Oatp1b3, and a water channel, Aqp1. We first show that the expression of Aqp1 amplifies MRI contrast in cultured cells engineered to express Oatp1b3. We demonstrate that the contrast amplification is caused by Aqp1-driven increase in water exchange, which provides the gadolinium ions internalized by Oatp1b3-expressing cells with access to a larger water pool compared with exchange-limited conditions. We further show that our methodology allows cells to be detected using approximately 10-fold lower concentrations of gadolinium than that in the Aqp1-free scenario. Finally, we show that our approach enables the imaging of mixed-cell cultures containing a low fraction of Oatp1b3-labeled cells that are undetectable on the basis of Oatp1b3 expression alone.
Keywords: MRI; Oatp1; aquaporins; diffusion; reporter genes.
© 2024 Wiley-VCH GmbH.
Conflict of interest statement
Conflicts of Interest
There are no conflicts to declare.
Figures

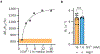
Update of
-
A dual-gene reporter-amplifier architecture for enhancing the sensitivity of molecular MRI by water exchange.bioRxiv [Preprint]. 2024 Jan 25:2024.01.22.576672. doi: 10.1101/2024.01.22.576672. bioRxiv. 2024. Update in: Chembiochem. 2024 May 17;25(10):e202400087. doi: 10.1002/cbic.202400087. PMID: 38328134 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

