trans-IV restriction: a new configuration for metal bis-cyclam complexes as potent CXCR4 inhibitors
- PMID: 38439632
- PMCID: PMC10949960
- DOI: 10.1039/d3dt01729j
trans-IV restriction: a new configuration for metal bis-cyclam complexes as potent CXCR4 inhibitors
Abstract
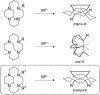
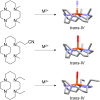
The chemokine receptor CXCR4 is implicated in multiple diseases including inflammatory disorders, cancer growth and metastasis, and HIV/AIDS. CXCR4 targeting has been evaluated in treating cancer metastasis and therapy resistance. Cyclam derivatives, most notably AMD3100 (Plerixafor™), are a common motif in small molecule CXCR4 antagonists. However, AMD3100 has not been shown to be effective in cancer treatment as an individual agent. Configurational restriction and transition metal complex formation increases receptor binding affinity and residence time. In the present study, we have synthesized novel trans-IV locked cyclam-based CXCR4 inhibitors, a previously unexploited configuration, and demonstrated their higher affinity for CXCR4 binding and CXCL12-mediated signaling inhibition compared to AMD3100. These results pave the way for even more potent CXCR4 inhibitors that may provide significant efficacy in cancer therapy.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

