Mitochondrial ferritin alleviates ferroptosis in a kainic acid-induced mouse epilepsy model by regulating iron homeostasis: Involvement of nuclear factor erythroid 2-related factor 2
- PMID: 38439636
- PMCID: PMC10912846
- DOI: 10.1111/cns.14663
Mitochondrial ferritin alleviates ferroptosis in a kainic acid-induced mouse epilepsy model by regulating iron homeostasis: Involvement of nuclear factor erythroid 2-related factor 2
Abstract
Background: Epilepsy is a widespread and chronic disease of the central nervous system caused by a variety of factors. Mitochondrial ferritin (FtMt) refers to ferritin located within the mitochondria that may protect neurons against oxidative stress by binding excess free iron ions in the cytoplasm. However, the potential role of FtMt in epilepsy remains unclear. We aimed to investigate whether FtMt and its related mechanisms can regulate epilepsy by modulating ferroptosis.
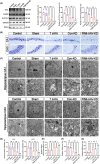
Methods: Three weeks after injection of adeno-associated virus (AAV) in the skull of adult male C57BL/6 mice, kainic acid (KA) was injected into the hippocampus to induce seizures. Primary hippocampal neurons were transfected with siRNA using a glutamate-mediated epilepsy model. After specific treatments, Western blot analysis, immunofluorescence, EEG recording, transmission electron microscopy, iron staining, silver staining, and Nissl staining were performed.
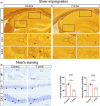
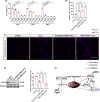
Results: At different time points after KA injection, the expression of FtMt protein in the hippocampus of mice showed varying degrees of increase. Knockdown of the FtMt gene by AAV resulted in an increase in intracellular free iron levels and a decrease in the function of iron transport-related proteins, promoting neuronal ferroptosis and exacerbating epileptic brain activity in the hippocampus of seizure mice. Additionally, increasing the expression level of FtMt protein was achieved by AAV-mediated upregulation of nuclear factor erythroid 2-related factor 2 (Nrf2) gene in the hippocampus of seizure mice.
Conclusions: In epilepsy, Nrf2 modulates ferroptosis by involving the expression of FtMt and may be a potential therapeutic mechanism of neuronal injury after epilepsy. Targeting this relevant process for treatment may be a therapeutic strategy to prevent epilepsy.
Keywords: Ferroptosis; FtMt; Nrf2; epilepsy; iron homeostasis.
© 2024 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there is no conflict of interest in the publication of this article.
Figures





References
-
- Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475‐482. - PubMed
-
- Du K, He M, Zhao D, et al. Mechanism of cell death pathways in status epilepticus and related therapeutic agents. Biomed Pharmacother. 2022;149:112875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

