A comparison of the epidemiology of kidney replacement therapy between Europe and the United States: 2021 data of the ERA Registry and the USRDS
- PMID: 38439701
- PMCID: PMC11483573
- DOI: 10.1093/ndt/gfae040
A comparison of the epidemiology of kidney replacement therapy between Europe and the United States: 2021 data of the ERA Registry and the USRDS
Abstract
Background: This paper compares the most recent data on the incidence and prevalence of kidney replacement therapy (KRT), kidney transplantation rates, and mortality on KRT from Europe to those from the United States (US), including comparisons of treatment modalities (haemodialysis (HD), peritoneal dialysis (PD), and kidney transplantation (KTx)).
Methods: Data were derived from the annual reports of the European Renal Association (ERA) Registry and the United States Renal Data System (USRDS). The European data include information from national and regional renal registries providing the ERA Registry with individual patient data. Additional analyses were performed to present results for all participating European countries together.
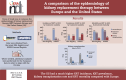
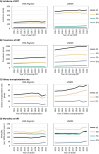
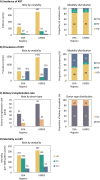
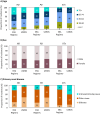
Results: In 2021, the KRT incidence in the US (409.7 per million population (pmp)) was almost 3-fold higher than in Europe (144.4 pmp). Despite the substantial difference in KRT incidence, approximately the same proportion of patients initiated HD (Europe: 82%, US: 84%), PD (14%; 13%, respectively), or underwent pre-emptive KTx (4%; 3%, respectively). The KRT prevalence in the US (2436.1 pmp) was 2-fold higher than in Europe (1187.8 pmp). Within Europe, approximately half of all prevalent patients were living with a functioning graft (47%), while in the US, this was one third (32%). The number of kidney transplantations performed was almost twice as high in the US (77.0 pmp) compared to Europe (41.6 pmp). The mortality of patients receiving KRT was 1.6-fold higher in the US (157.3 per 1000 patient years) compared to Europe (98.7 per 1000 patient years).
Conclusions: The US had a much higher KRT incidence, prevalence, and mortality compared to Europe, and despite a higher kidney transplantation rate, a lower proportion of prevalent patients with a functioning graft.
Keywords: epidemiology; incidence; kidney replacement therapy; mortality; prevalence.
© The Author(s) 2024. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
V.S.S. reports research funding from the European Renal Association, outside the submitted work. R.S. reports travel support from Chiesi, Amicus, Sanofi Genzyme and Takeda, outside the submitted work. M.F.S.R. reports consulting fees from Baxter, Fresenius and Nipro; lecture fees from Baxter and Fresenius; travel grants from Vifor, Fresenius and Palex; and membership of the Fresenius European Home Dialysis Advisory Board, outside the submitted work. S.B. reports consulting fees from GSK and Astra Zeneca, outside the submitted work. M.L. reports membership of the Serbian Society of Nephrology board, outside the submitted work. H.R. reports membership of BANTAO, MKS and EAPE boards, outside the submitted work. N.A.-F. reports lecture fees from Astra-Zeneca, Vifor Pharma, Boehringer-Ingelheim and Bayer; travel grants from Vifor Pharma; and membership of Astra Zeneca and the Andalusian Nephrology Society boards, all outside the submitted work. P.F. reports grants from Finska läkaresällskapet and Liv och Hälsa and consulting fees from Baxter, Astra Zeneca, GSK and Astellas, and membership of a Baxter board, outside the submitted work. D.N. reports board membership of the UK Kidney Association, outside the submitted work. K.J.J. reports research funding from the European Renal Association and the European Society for Paediatric Nephrology; and board membership from the SHare RR working group (ISN), outside the submitted work. A.O. reports grants from Sanofi, consultancy or speaker fees or travel support from Advicciene, Astellas, Astrazeneca, Amicus, Amgen, Boehringer Ingelheim, Fresenius Medical Care, GSK, Bayer, Sanofi-Genzyme, Menarini, Mundipharma, Kyowa Kirin, Lilly, Alexion, Freeline, Idorsia, Chiesi, Otsuka, Novo-Nordisk, Sysmex, and Vifor Fresenius Medical Care Renal Pharma; Directorship of the Catedra Mundipharma-UAM of diabetic kidney disease and the Catedra Astrazeneca-UAM of chronic kidney disease and electrolytes; membership of the European Renal Association board; and stock options from Telara Farma, all outside the submitted work. R.B., M.E.A., B.A.B., D.R., J.C.R.S.M., P.U.M., M.A.G.J.D., O.L.R.A., J.T.B., M.L., S.T.A., O.S.I., M.A., M.R.C., K.H., G.M., J.K., P.M.A., and A.K. declare that they have no relevant conflicts of interests.
Figures

References
-
- United States Renal Data System . 2023 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2023. Available from: https://usrds-adr.niddk.nih.gov/2023.
-
- European Statistical Office (Eurostat) . Database—Population and Social Conditions. https://ec.europa.eu/eurostat/data/database (12 January 2023, date last accessed).
-
- Centers for Disease Control and Prevention . Kidney disease surveillance system: trends in prevalence of CKD stages among U.S. Adults. Available from: https://nccd.cdc.gov/ckd/detail.aspx?Qnum=Q372 (5 January 2023, date last accessed).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

