Multiomics profiling of urothelial carcinoma in situ reveals CIS-specific gene signature and immune characteristics
- PMID: 38439961
- PMCID: PMC10910238
- DOI: 10.1016/j.isci.2024.109179
Multiomics profiling of urothelial carcinoma in situ reveals CIS-specific gene signature and immune characteristics
Abstract
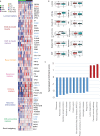
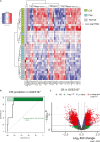
Urothelial carcinoma in situ (CIS) is an aggressive phenotype of non-muscle-invasive bladder cancer. Molecular features unique to CIS compared to high-grade papillary tumors are underexplored. RNA sequencing of CIS, papillary tumors, and normal urothelium showed lower immune marker expression in CIS compared to papillary tumors. We identified a 46-gene expression signature in CIS samples including selectively upregulated known druggable targets MTOR, TYK2, AXIN1, CPT1B, GAK, and PIEZO1 and selectively downregulated BRD2 and NDUFB2. High expression of selected genes was significantly associated with CIS in an independent dataset. Mutation analysis of matched CIS and papillary tumors revealed shared mutations between samples across time points and mutational heterogeneity. CCDC138 was the most frequently mutated gene in CIS. The immunological landscape showed higher levels of PD-1-positive cells in CIS lesions compared to papillary tumors. We identified CIS lesions to have distinct characteristics compared to papillary tumors potentially contributing to the aggressive phenotype.
Keywords: Cancer; Immunology; Omics.
© 2024 The Authors.
Conflict of interest statement
L.D. has sponsored research agreements with C2i Genomics, Natera, AstraZeneca, Photocure, and Ferring and has an advisory/consulting role at Ferring, MSD, and UroGen. L.D. has received speaker honoraria from AstraZeneca, Pfizer, and Roche and received travel support from MSD. L.D. is a board member at BioXpedia. S.P.L. has funding for clinical trials from Aura Bioscience, FKD, JBL (SWOG), Genentech (SWOG), Merck (Alliance), QED Therapeutics, Surge Therapeutics, Vaxiion, and Viventia; is a consultant/Advisory Board member for Aura Bioscience, BMS, Pfizer/EMD Serono, Protara, Surge Therapeutics, Vaxiion, and Verity; has a patent for the TCGA classifier; and received honoraria from Grand Rounds Urology and UroToday. J.B.J. is a member of Advisory Boards at Ferring, Roche, Cepheid, Urotech, Olympus, AMBU, Janssen, and Cystotech; is a speaker at medac, Olympus, Intuitive Surgery, and Photocure ASA; and has research collaborations with Medac, Photocure ASA, Roche, Ferring, Olympus, Intuitive Surgery, Astellas, Cepheid, Nucleix, Urotech, Pfizer, AstraZenica, MeqNordic, Laborie, VingMed, AMBU, and Cystotech. H.A.-A. provides consultation to AstaZeneca and Paige.AI. B.I. runs clinical trials or scientific studies in collaboration with FKD Therapies, Taris Biomedical, CG Oncology, Genentech Inc, Janssen, Medtronic, and Profound Medical and has consultant or advisory roles at Seattle Genetics, Combat Medical, and Johnson & Johnson.
Figures




References
-
- Lamm D.L., van der Meijden P.M., Morales A., Brosman S.A., Catalona W.J., Herr H.W., Soloway M.S., Steg A., Debruyne F.M. Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer. J. Urol. 1992;147:596–600. doi: 10.1016/s0022-5347(17)37316-0. - DOI - PubMed
-
- McKenney J.K., Gomez J.A., Desai S., Lee M.W., Amin M.B. Morphologic expressions of urothelial carcinoma in situ: a detailed evaluation of its histologic patterns with emphasis on carcinoma in situ with microinvasion. Am. J. Surg. Pathol. 2001;25:356–362. doi: 10.1097/00000478-200103000-00010. - DOI - PubMed
-
- Epstein J.I., Amin M.B., Reuter V.R., Mostofi F.K. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am. J. Surg. Pathol. 1998;22:1435–1448. doi: 10.1097/00000478-199812000-00001. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

