Novel role of lncRNAs regulatory network in papillary thyroid cancer
- PMID: 38440062
- PMCID: PMC10909982
- DOI: 10.1016/j.bbrep.2024.101674
Novel role of lncRNAs regulatory network in papillary thyroid cancer
Abstract
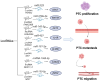
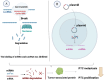
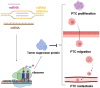
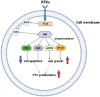
Papillary thyroid cancer (PTC) is the most common endocrine malignancy. The incidence of PTC has increased annually worldwide. Thus, PTC diagnosis and treatment attract more attention. Noncoding RNAs (lncRNAs) play crucial roles in PTC progression and act as prognostic biomarkers. Moreover, microRNAs (miRNAs) and epithelial-mesenchymal transition (EMT)-associated proteins have potential biomarkers for diagnosing and treating PTC. However, the correlation of lncRNAs with miRNAs and EMT-associated proteins needs further clarification. The present review highlights the recent advances of lncRNAs in PTC. We significantly summarized the two molecular regulatory mechanisms in PTC progress, including lncRNAs-miRNAs-protein signaling axes and lncRNAs-EMT pathways. This review will help our understanding of the association between lncRNAs and PTC and may assist us in evaluating the prognosis for PTC patients. Taken together, targeting the lncRNAs regulatory network has promising applications in diagnosing and treating PTC.
Keywords: Diagnosis; EMT; Papillary thyroid cancer; Prognosis; lncRNAs; miRNAs.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
A narrative review of papillary thyroid carcinoma-related long non-coding RNAs and their relevance to malignant tumors.Transl Cancer Res. 2025 Mar 30;14(3):2125-2149. doi: 10.21037/tcr-24-1038. Epub 2025 Mar 27. Transl Cancer Res. 2025. PMID: 40224997 Free PMC article. Review.
-
Noncoding RNAs in Papillary Thyroid Cancer: Interaction with Cancer-Associated Fibroblasts (CAFs) in the Tumor Microenvironment (TME) and Regulators of Differentiation and Lymph Node Metastasis.Adv Exp Med Biol. 2021;1350:145-155. doi: 10.1007/978-3-030-83282-7_7. Adv Exp Med Biol. 2021. PMID: 34888848
-
Identification of Hub lncRNAs Along With lncRNA-miRNA-mRNA Network for Effective Diagnosis and Prognosis of Papillary Thyroid Cancer.Front Pharmacol. 2021 Oct 13;12:748867. doi: 10.3389/fphar.2021.748867. eCollection 2021. Front Pharmacol. 2021. PMID: 34721037 Free PMC article.
-
Epigenetic regulation of papillary thyroid carcinoma by long non-coding RNAs.Semin Cancer Biol. 2022 Aug;83:253-260. doi: 10.1016/j.semcancer.2021.03.027. Epub 2021 Mar 27. Semin Cancer Biol. 2022. PMID: 33785446 Review.
-
Integrated analysis of long noncoding RNA interactions reveals the potential role in progression of human papillary thyroid cancer.Cancer Med. 2018 Nov;7(11):5394-5410. doi: 10.1002/cam4.1721. Epub 2018 Oct 14. Cancer Med. 2018. PMID: 30318850 Free PMC article.
Cited by
-
A narrative review of papillary thyroid carcinoma-related long non-coding RNAs and their relevance to malignant tumors.Transl Cancer Res. 2025 Mar 30;14(3):2125-2149. doi: 10.21037/tcr-24-1038. Epub 2025 Mar 27. Transl Cancer Res. 2025. PMID: 40224997 Free PMC article. Review.
-
Causal associations between circulating protein ratios and drug resistance in papillary thyroid cancer: a Mendelian randomization study.Discov Oncol. 2025 Jan 4;16(1):4. doi: 10.1007/s12672-025-01758-2. Discov Oncol. 2025. PMID: 39754686 Free PMC article.
References
-
- Jung C.K., Little M.P., Lubin J.H., Brenner A.V., Wells S.A., Jr., Sigurdson A.J., Nikiforov Y.E. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J. Clin. Endocrinol. Metab. 2014;99(2):E276–E285. - PMC - PubMed
-
- Vaccarella S., Franceschi S., Bray F., Wild C.P., Plummer M., Dal Maso L. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N. Engl. J. Med. 2016;375(7):614–617. - PubMed
-
- Megwalu U.C., Moon P.K. Thyroid cancer incidence and mortality trends in the United States: 2000-2018. Thyroid. 2022;32(5):560–570. - PubMed
-
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

