The expanding roles of Nr6a1 in development and evolution
- PMID: 38440075
- PMCID: PMC10909835
- DOI: 10.3389/fcell.2024.1357968
The expanding roles of Nr6a1 in development and evolution
Abstract
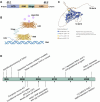
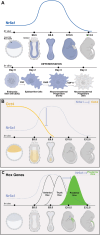
The Nuclear Receptor (NR) family of transcriptional regulators possess the ability to sense signalling molecules and directly couple that to a transcriptional response. While this large class of proteins are united by sequence and structural homology, individual NR functional output varies greatly depending on their expression, ligand selectivity and DNA binding sequence specificity. Many NRs have remained somewhat enigmatic, with the absence of a defined ligand categorising them as orphan nuclear receptors. One example is Nuclear Receptor subfamily 6 group A member 1 (Nr6a1), an orphan nuclear receptor that has no close evolutionary homologs and thus is alone in subfamily 6. Nonetheless, Nr6a1 has emerged as an important player in the regulation of key pluripotency and developmental genes, as functionally critical for mid-gestational developmental progression and as a possible molecular target for driving evolutionary change in animal body plan. Here, we review the current knowledge on this enigmatic nuclear receptor and how it impacts development and evolution.
Keywords: GCNF; Hox genes; Nr6a1; Oct4; axial elongation; orphan nuclear receptor; retinoic acid.
Copyright © 2024 Li, Mascarinas and McGlinn.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Revisiting the role of GCNF in embryonic development.Semin Cell Dev Biol. 2013 Dec;24(10-12):679-86. doi: 10.1016/j.semcdb.2013.08.003. Epub 2013 Sep 9. Semin Cell Dev Biol. 2013. PMID: 24029702 Review.
-
Orphan nuclear receptor GCNF is required for the repression of pluripotency genes during retinoic acid-induced embryonic stem cell differentiation.Mol Cell Biol. 2005 Oct;25(19):8507-19. doi: 10.1128/MCB.25.19.8507-8519.2005. Mol Cell Biol. 2005. PMID: 16166633 Free PMC article.
-
A Structural Investigation into Oct4 Regulation by Orphan Nuclear Receptors, Germ Cell Nuclear Factor (GCNF), and Liver Receptor Homolog-1 (LRH-1).J Mol Biol. 2016 Dec 4;428(24 Pt B):4981-4992. doi: 10.1016/j.jmb.2016.10.025. Epub 2016 Oct 27. J Mol Biol. 2016. PMID: 27984042 Free PMC article.
-
Fine mapping of a swine quantitative trait locus for number of vertebrae and analysis of an orphan nuclear receptor, germ cell nuclear factor (NR6A1).Genome Res. 2007 May;17(5):586-93. doi: 10.1101/gr.6085507. Epub 2007 Apr 6. Genome Res. 2007. PMID: 17416745 Free PMC article.
-
The ROR nuclear orphan receptor subfamily: critical regulators of multiple biological processes.Prog Nucleic Acid Res Mol Biol. 2001;69:205-47. doi: 10.1016/s0079-6603(01)69048-2. Prog Nucleic Acid Res Mol Biol. 2001. PMID: 11550795 Review.
Cited by
-
Bioinformatic approach to identifying causative missense polymorphisms in animal genomes.BMC Genomics. 2024 Dec 19;25(1):1226. doi: 10.1186/s12864-024-11126-z. BMC Genomics. 2024. PMID: 39701989 Free PMC article.
References
-
- Bauer U. M., Schneider-Hirsch S., Reinhardt S., Pauly T., Maus A., Wang F., et al. (1997). Neuronal cell nuclear factor--a nuclear receptor possibly involved in the control of neurogenesis and neuronal differentiation. Eur. J. Biochem. 249, 826–837. 10.1111/j.1432-1033.1997.t01-1-00826.x - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

