Priority setting: women's health topics in multiple sclerosis
- PMID: 38440114
- PMCID: PMC10910071
- DOI: 10.3389/fneur.2024.1355817
Priority setting: women's health topics in multiple sclerosis
Abstract
Background: A scoping review found that most studies on women's health in multiple sclerosis (MS) focused on pregnancy, fetal/neonatal outcomes and sexual dysfunction. Few studies addressed menopause, contraception, gynecologic cancers/cancer screening. However, the perceived relative importance of these knowledge gaps to people living with MS and other partners is unknown. We engaged a range of partners, including people living with MS, health care providers, researchers, and patient advocacy groups, to set priorities for future research in women's health in MS.
Methods: We employed a three-step global engagement process. First, we identified which broad research topics relevant to women's health in MS were of highest priority using two surveys. Second, we developed specific research questions within these topics using focus groups. Finally, we prioritized the research questions with a third survey.
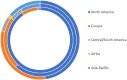
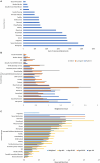
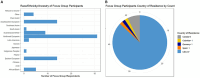
Results: Overall, 5,266 individuals responded to the initial surveys [n = 1,430 global survey, mean (SD) age 50.0 (12.6), all continents; n = 3,836 North American Research Committee on Multiple Sclerosis survey, mean (SD) age 64.8 (9.6), United States]. Menopause, sexual dysfunction, pregnancy, gynecologic cancer/cancer screening, hormones and parenthood were identified as the most important topics. Focus groups generated 80 potential research questions related to these topics. In the final survey 712 individuals prioritized these questions. The highest priority questions in each research topic were: (i) How do perimenopause and menopause affect disease activity, course, response to disease-modifying treatment and quality of life in MS; (ii) What are the most effective strategies for managing issues around sexual intimacy, including related to low sexual desire, changes in physical function, and MS symptoms; (iii) Are there long-term effects of disease-modifying therapies on the children of persons with MS; (iv) What are the short and long-term effects of disease-modifying drugs on gynecologic cancer risk, particularly for high efficacy disease-modifying drugs and hematopoietic stem cell transplantation; (v) Are there hormone related treatments that can stabilize fluctuations in MS symptoms; and (vi) How does MS fatigue impact parenting strategies.
Conclusion: Priorities for research relating to women's health issues for persons with MS have been delineated using a collaborative process with key partners. Alignment of future research with these priorities should be monitored.
Keywords: cancer; focus groups; menopause; multiple sclerosis; survey; women’s health.
Copyright © 2024 Ross, Finlayson, Amato, Cohen, Hellwig, Tintore, Vukusic, Salter and Marrie.
Conflict of interest statement
RM receives research funding from: CIHR, Research Manitoba, MS Canada, Crohn’s and Colitis Canada, National Multiple Sclerosis Society, CMSC, the Arthritis Society and the US Department of Defense, and is a co-investigator on studies receiving funding from Biogen Idec and Roche Canada. She holds the Waugh Family Chair in Multiple Sclerosis. LR received research funding from the National Multiple Sclerosis Society. SV has received consulting and lecturing fees, travel grants and unconditional research support from Biogen, Janssen, Merck, Novartis, Roche, Sandoz and Sanofi. JC has received personal compensation for consulting for Astoria, Bristol-Myers Squibb, Convelo, EMD Serono, FiND Therapeutics, INMune, and Sandoz; and serving as an Editor of Multiple Sclerosis Journal. MF holds research funding from MS Canada, and has received consulting or teaching honoraria from Novartis and Biogen. KH received personal compensation as a speaker for Biogen, Bristol-Myers Squibb, Roche, Teva, Novartis, Bayer, Sanofi-Genzyme and Merck. She receives currently funding by the Innovations fonds, the National MS society, Biogen, Teva, Novartis, Roche, Sanofi-Genzyme and Merck. MT has received compensation for consulting services, speaking honoraria and research support from Almirall, Bayer Schering Pharma, Biogen-Idec, Genzyme, Janssen, Merck-Serono, Novartis, Roche, Sanofi-Aventis, Viela Bio and Teva Pharmaceuticals. Data Safety Monitoring Board for Parexel and UCB Biopharma, Relapse Adjudication Committee for IMCYSE SA. MA has received consulting and lecturing fees, travel grants and unconditional research support from Biogen, Janssen, Merck, Novartis, Roche, Sandoz, Sanofi and Celgene BMS. AS receives research funding from Multiple Sclerosis Society of Canada, National Multiple Sclerosis Society, Consortium of MS Centers and the US Department of Defense and is a member of editorial board for Neurology. She serves as a consultant for Gryphon Bio, LLC and Abata Therapeutics. She is a member of the Data and Safety Monitoring Board for Premature Infants Receiving Milking or Delayed Cord Clamping (PREMOD2), Central Vein Sign: A Diagnostic Biomarker in Multiple Sclerosis (CAVS-MS), and Methotrexate treatment of Arthritis caused by Chikungunya virus (MARCH). The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
-
- Cowan K, Oliver S. The James Lind Alliance guidebook. Southampton, UK: National Institute for Health Research Evaluation, Trials and Studies Coordinating Centre; (2013).
Grants and funding
LinkOut - more resources
Full Text Sources

