Varenicline solution nasal spray for dry eye disease in Chinese patients: a randomized phase 3 trial
- PMID: 38440130
- PMCID: PMC10909742
- DOI: 10.1016/j.lanwpc.2024.101032
Varenicline solution nasal spray for dry eye disease in Chinese patients: a randomized phase 3 trial
Abstract
Background: Dry eye disease has a high prevalence and exerts a significant negative effect on quality of life. In China, there are currently no available nasal sprays to promote natural tear production in patients with dry eye disease. We therefore evaluated the efficacy and safety of OC-01 (varenicline solution) nasal spray versus vehicle in Chinese patients with dry eye disease.
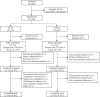
Methods: This was a randomized, multicenter, double-masked, vehicle-controlled, phase 3 clinical trial conducted at ophthalmology departments in 20 hospitals across China (NCT05378945). Eligible patients had a diagnosis of dry eye disease based on patient symptoms, Eye Dryness Score (EDS), Schirmer's Test (with topical anesthesia) Score (STS), and corneal fluorescein staining (CFS) score. Participants were randomly assigned 1:1 using an Interactive Web Response System (IWRS) to receive OC-01 0.6 mg/mL twice daily (BID) or vehicle nasal spray. Participants, investigators, and sponsor were all masked to treatment assignment. The primary endpoint was the percentage of subjects in the intention-to-treat population achieving ≥10 mm improvement in STS from baseline at week 4.
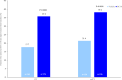
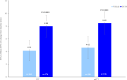
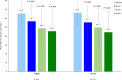
Findings: In total, 340 patients were randomized from 21 July 2022 to 04 April 2023, 78.8% were female. Patients in the OC-01 group (n = 176) had significantly higher achievement of ≥10 mm improvement in STS (35.8% [n = 63] versus 17.7% [n = 29], stratified odds ratio: 2.67, 95% CI: 1.570-4.533, p = 0.0002) and a significantly greater increase from baseline STS (least-squares mean difference [SE]: 3.87 [0.794], p < 0.0001) at week 4 versus the vehicle group (n = 164). In addition, OC-01 led to a numerically greater reduction in mean EDS from baseline at week 4 compared to the vehicle group (LS mean [SE] difference: -1.3 [2.20]; 95% CI: -5.64 to 2.99, p = 0.5467). The most common adverse event was mild, transient sneezing (78% of OC-01 administrations). No serious adverse events related to nasal administration occurred.
Interpretation: OC-01 (varenicline solution) nasal spray BID has clinically meaningful efficacy for reducing the signs (as measured by STS) and may improve the symptoms (as measured by EDS) of dry eye disease, with an excellent safety and tolerability profile, in the Chinese population.
Funding: Jixing Pharmaceutical Co. Ltd.
Keywords: Dry eye disease; Eye dryness score; Nicotinic acetylcholine receptor agonist; Randomized clinical trial; Schirmer's test score; Varenicline nasal spray.
© 2024 The Author(s).
Conflict of interest statement
No conflicts of interest to declare.
Figures
Similar articles
-
Efficacy and Safety of OC-01 (Varenicline Solution) Nasal Spray on Signs and Symptoms of Dry Eye Disease: The ONSET-2 Phase 3 Randomized Trial.Ophthalmology. 2022 Apr;129(4):379-387. doi: 10.1016/j.ophtha.2021.11.004. Epub 2021 Nov 10. Ophthalmology. 2022. PMID: 34767866 Clinical Trial.
-
Efficacy and Safety of Single-dose OC-02 (Simpinicline Solution) Nasal Spray on Signs and Symptoms of Dry Eye Disease: The PEARL Phase II Randomized Trial.Clin Ther. 2022 Sep;44(9):1178-1186. doi: 10.1016/j.clinthera.2022.07.006. Epub 2022 Aug 11. Clin Ther. 2022. PMID: 35965109 Clinical Trial.
-
A phase II randomized trial to evaluate the long-term (12-week) efficacy and safety of OC-01 (varenicline solution) nasal spray for dry eye disease: The MYSTIC study.Ocul Surf. 2022 Apr;24:15-21. doi: 10.1016/j.jtos.2021.12.007. Epub 2021 Dec 15. Ocul Surf. 2022. PMID: 34920097 Clinical Trial.
-
Varenicline Solution Nasal Spray: A Review in Dry Eye Disease.Drugs. 2022 Sep;82(14):1481-1488. doi: 10.1007/s40265-022-01782-4. Epub 2022 Oct 5. Drugs. 2022. PMID: 36197638 Free PMC article. Review.
-
The efficacy and safety of varenicline nasal spray for the management of dry eye signs: a systematic review and meta-analysis.BMC Ophthalmol. 2023 Jul 14;23(1):319. doi: 10.1186/s12886-023-03069-y. BMC Ophthalmol. 2023. PMID: 37452334 Free PMC article.
Cited by
-
Varenicline Solution Nasal Spray 0.03 Mg for the Treatment of Dry Eye Disease Following Photorefractive Keratectomy.Clin Ophthalmol. 2024 Oct 5;18:2777-2784. doi: 10.2147/OPTH.S474747. eCollection 2024. Clin Ophthalmol. 2024. PMID: 39386175 Free PMC article. Clinical Trial.
References
-
- Dry eye association China chapter, ocular surface and tear disease group of the ophthalmology professional committee of the cross-strait medical and health exchange association, ocular surface and dry eye group of the ophthalmologist branch of the Chinese medical doctor association. Chinese dry eye expert consensus: definition and classification (2020) Chin J Ophthalmol. 2020;56(6):418–422.
-
- Craig J.P., Nichols K.K., Akpek E.K., et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. - PubMed
-
- Liu Z., Zhang X. Interpret the definition and classification of dry eye in the 2017 dry eye expert consensus of the international tear film and ocular surface association. Chin J Ophthalmol. 2018;54(4):246–248.
-
- Expert Consensus Expert Group on the Standardized Construction of Dry Eye Diagnosis and Treatment Centres. Dry Eye Rehabilitation Professional Group of Visual Rehabilitation Special Committee of Chinese Rehabilitation Medical Association Expert consensus on the standardization of dry eye diagnosis and treatment centres in China (2021) Chin J Exp Ophthalmol. 2021;39(6):473–476.
-
- Corneal Disease Group, Ophthalmology Branch of Chinese Medical Association Expert consensus on clinical diagnosis and treatment of dry eye (2013) Chin J Ophthalmol. 2013;49(1):73–75.
LinkOut - more resources
Full Text Sources
Miscellaneous

